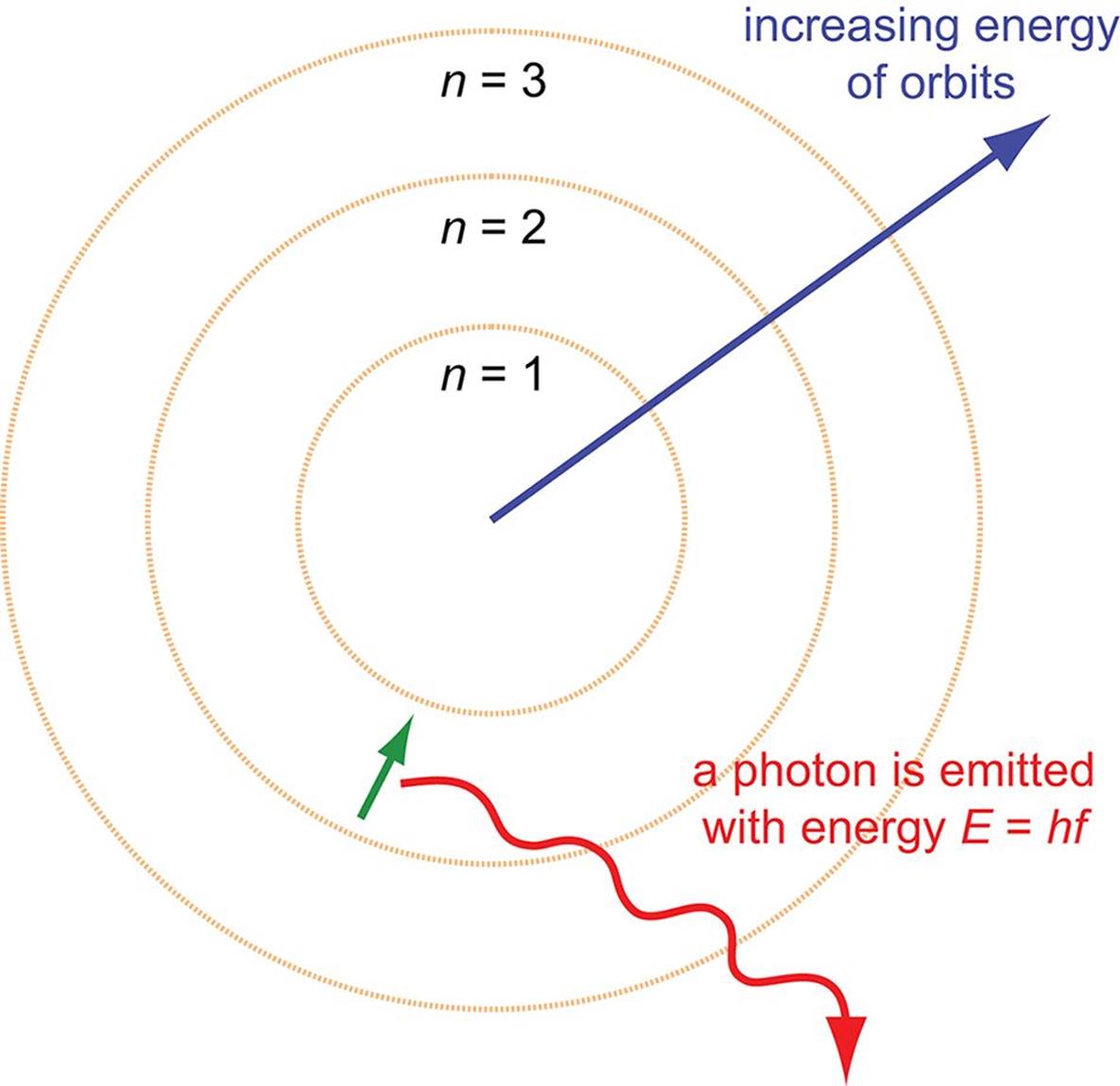
The Photon Can Be Absorbed By The Mercury Atom Because . as we will soon see, photons can be absorbed or emitted by atoms and molecules. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. The electrons in an atom can only occupy certain allowed energy levels. When a photon is absorbed, its. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. a strange implication of this experiment is that light can behave as a kind of massless particle now known as a photon whose. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the.
from schoolbag.info
so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. When a photon is absorbed, its. The electrons in an atom can only occupy certain allowed energy levels. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. as we will soon see, photons can be absorbed or emitted by atoms and molecules. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. a strange implication of this experiment is that light can behave as a kind of massless particle now known as a photon whose.
Rutherford, Planck, and Bohr Atomic Structure Review Training
The Photon Can Be Absorbed By The Mercury Atom Because so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. as we will soon see, photons can be absorbed or emitted by atoms and molecules. The electrons in an atom can only occupy certain allowed energy levels. a strange implication of this experiment is that light can behave as a kind of massless particle now known as a photon whose. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. When a photon is absorbed, its.
From ar.inspiredpencil.com
Difference Between Photons And Electrons The Photon Can Be Absorbed By The Mercury Atom Because in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. as we will soon see, photons can be absorbed or emitted by atoms and molecules. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. a strange. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.researchgate.net
Interaction processes of incident Xray photons with atoms in the The Photon Can Be Absorbed By The Mercury Atom Because a strange implication of this experiment is that light can behave as a kind of massless particle now known as a photon whose. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. as we will soon see, photons can be absorbed or emitted by atoms and molecules.. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.slideserve.com
PPT Matter and Particles of Light Quantum Theory PowerPoint The Photon Can Be Absorbed By The Mercury Atom Because When a photon is absorbed, its. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. as we will soon see, photons can be absorbed or. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.astronoo.com
Principle of absorption and emission atomic — Astronoo The Photon Can Be Absorbed By The Mercury Atom Because as we will soon see, photons can be absorbed or emitted by atoms and molecules. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. When a photon is absorbed, its. The electrons in an atom can only occupy certain allowed energy levels. a strange implication of. The Photon Can Be Absorbed By The Mercury Atom Because.
From chemcollective.org
CHEM1315 Lab 8 Atomic Spectrum The Photon Can Be Absorbed By The Mercury Atom Because When a photon is absorbed, its. so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. The electrons in an atom. The Photon Can Be Absorbed By The Mercury Atom Because.
From chem.libretexts.org
14.1 Vocabulary Chemistry LibreTexts The Photon Can Be Absorbed By The Mercury Atom Because as we will soon see, photons can be absorbed or emitted by atoms and molecules. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. When a photon is absorbed, its. . The Photon Can Be Absorbed By The Mercury Atom Because.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube The Photon Can Be Absorbed By The Mercury Atom Because When a photon is absorbed, its. as we will soon see, photons can be absorbed or emitted by atoms and molecules. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. A photon may be absorbed by a particle, such as the electron, transferring energy from. The Photon Can Be Absorbed By The Mercury Atom Because.
From in.pinterest.com
Photon Easy Science Light energy science, Light energy, Easy science The Photon Can Be Absorbed By The Mercury Atom Because The electrons in an atom can only occupy certain allowed energy levels. so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. a. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.pinterest.com
Light and photosynthetic pigments The lightdependent reactions The Photon Can Be Absorbed By The Mercury Atom Because photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. When a photon is absorbed, its. so a photon can be absorbed by an atom by. The Photon Can Be Absorbed By The Mercury Atom Because.
From general.chemistrysteps.com
Calculating The Energy of a Photon Chemistry Steps The Photon Can Be Absorbed By The Mercury Atom Because a strange implication of this experiment is that light can behave as a kind of massless particle now known as a photon whose. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. so a photon can be absorbed by an atom by kicking an electron to a higher energy level. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.youtube.com
Quantized Energy and Photons Chemistry Tutorial YouTube The Photon Can Be Absorbed By The Mercury Atom Because A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. The electrons in an atom can only occupy certain allowed energy levels. a strange implication of this experiment is that light can. The Photon Can Be Absorbed By The Mercury Atom Because.
From slidetodoc.com
Basics of Biological Effects of Ionizing Radiation Lecture The Photon Can Be Absorbed By The Mercury Atom Because A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. so. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.slideserve.com
PPT Interaction of electrons with matter Interaction of photons with The Photon Can Be Absorbed By The Mercury Atom Because as we will soon see, photons can be absorbed or emitted by atoms and molecules. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if. The Photon Can Be Absorbed By The Mercury Atom Because.
From amazingsciencefacts.com
What Is A Photon? Amazing Science Facts The Photon Can Be Absorbed By The Mercury Atom Because as we will soon see, photons can be absorbed or emitted by atoms and molecules. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. a strange implication. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.slideserve.com
PPT Chapter 38 Photons and Matter Waves PowerPoint Presentation, free The Photon Can Be Absorbed By The Mercury Atom Because so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. photons can be absorbed or emitted only by atoms and molecules that. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.physicstutoronline.co.uk
Photoelectric Effect explained in this fully illustrated article The Photon Can Be Absorbed By The Mercury Atom Because When a photon is absorbed, its. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. as we will soon see, photons can be absorbed or emitted by atoms and molecules. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. . The Photon Can Be Absorbed By The Mercury Atom Because.
From www.youtube.com
A photon of `300 nm` is absorbed by a gas which then reemits photon The Photon Can Be Absorbed By The Mercury Atom Because The electrons in an atom can only occupy certain allowed energy levels. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that. The Photon Can Be Absorbed By The Mercury Atom Because.
From file.scirp.org
The SpinSpin Interaction and the New Concept of Photon The Photon Can Be Absorbed By The Mercury Atom Because thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. a strange implication of this experiment is that light can behave as a kind of massless particle now known as a photon whose. photons can be absorbed or emitted only by atoms and molecules that have precisely the. The Photon Can Be Absorbed By The Mercury Atom Because.
From philschatz.com
Photon Momentum · Physics The Photon Can Be Absorbed By The Mercury Atom Because thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. When a photon is absorbed, its. The electrons in an atom can only occupy certain allowed energy levels. . The Photon Can Be Absorbed By The Mercury Atom Because.
From www.sciencenews.org
Colliding photons made matter. But are the photons ‘real’? Science News The Photon Can Be Absorbed By The Mercury Atom Because A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. When a photon is absorbed, its. The electrons in an atom can only occupy certain allowed energy levels. in. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.livescience.com
Photoelectric Effect Explanation & Applications Live Science The Photon Can Be Absorbed By The Mercury Atom Because The electrons in an atom can only occupy certain allowed energy levels. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. When a photon is absorbed,. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.nagwa.com
Question Video Finding the Required Wavelength of an Absorbed Photon The Photon Can Be Absorbed By The Mercury Atom Because photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave.. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.chegg.com
Solved As a mercury atom absorbs a photon of energy, an The Photon Can Be Absorbed By The Mercury Atom Because thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. The electrons in an atom can only occupy certain allowed energy levels. so a photon can be absorbed. The Photon Can Be Absorbed By The Mercury Atom Because.
From studylib.net
phys586lec07photons1 The Photon Can Be Absorbed By The Mercury Atom Because so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. When a photon is absorbed, its. The electrons in an atom can only occupy certain allowed energy. The Photon Can Be Absorbed By The Mercury Atom Because.
From studylib.net
Absorption / Emission of Photons and Conservation of Energy The Photon Can Be Absorbed By The Mercury Atom Because as we will soon see, photons can be absorbed or emitted by atoms and molecules. a strange implication of this experiment is that light can behave as a kind of massless particle now known as a photon whose. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. The electrons in. The Photon Can Be Absorbed By The Mercury Atom Because.
From users.highland.edu
Atomic Spectra and Models of the Atom The Photon Can Be Absorbed By The Mercury Atom Because thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. photons. The Photon Can Be Absorbed By The Mercury Atom Because.
From rwu.pressbooks.pub
Chapter 12. Photosynthesis Introduction to Molecular and Cell Biology The Photon Can Be Absorbed By The Mercury Atom Because When a photon is absorbed, its. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. as we will soon see, photons can be absorbed or emitted by atoms and molecules. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.slideshare.net
Interaction of photons with matter The Photon Can Be Absorbed By The Mercury Atom Because so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. as we will soon see, photons can be absorbed or emitted by atoms and molecules. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.slideshare.net
Photon and energy levels The Photon Can Be Absorbed By The Mercury Atom Because in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. When a photon is absorbed, its. A photon may be absorbed by a particle, such as the electron, transferring. The Photon Can Be Absorbed By The Mercury Atom Because.
From schoolbag.info
Rutherford, Planck, and Bohr Atomic Structure Review Training The Photon Can Be Absorbed By The Mercury Atom Because as we will soon see, photons can be absorbed or emitted by atoms and molecules. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. thus the hydrogen atoms in. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.slideserve.com
PPT Chapter 5 Light PowerPoint Presentation, free download ID2768132 The Photon Can Be Absorbed By The Mercury Atom Because The electrons in an atom can only occupy certain allowed energy levels. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. thus the hydrogen atoms in the sample have absorbed energy from the electrical discharge and decayed from a higher. photons can be absorbed or emitted. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.chegg.com
Solved Electrically excited mercury atoms have particularly The Photon Can Be Absorbed By The Mercury Atom Because in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. as we will soon see, photons can be absorbed or emitted by atoms and molecules.. The Photon Can Be Absorbed By The Mercury Atom Because.
From www.tec-science.com
Bohr's atomic model tecscience The Photon Can Be Absorbed By The Mercury Atom Because The electrons in an atom can only occupy certain allowed energy levels. so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. as we will soon see, photons can be absorbed or emitted by atoms and molecules. thus the hydrogen atoms in. The Photon Can Be Absorbed By The Mercury Atom Because.
From energywavetheory.com
Photoelectric Effect EWT The Photon Can Be Absorbed By The Mercury Atom Because When a photon is absorbed, its. in this demonstration, light from a sodium source will be absorbed by sodium gas, because the photon energies have the. so a photon can be absorbed by an atom by kicking an electron to a higher energy level , if the photon has that energy. thus the hydrogen atoms in the. The Photon Can Be Absorbed By The Mercury Atom Because.
From ar.inspiredpencil.com
Electron Energy Levels Photon The Photon Can Be Absorbed By The Mercury Atom Because The electrons in an atom can only occupy certain allowed energy levels. a strange implication of this experiment is that light can behave as a kind of massless particle now known as a photon whose. as we will soon see, photons can be absorbed or emitted by atoms and molecules. A photon may be absorbed by a particle,. The Photon Can Be Absorbed By The Mercury Atom Because.