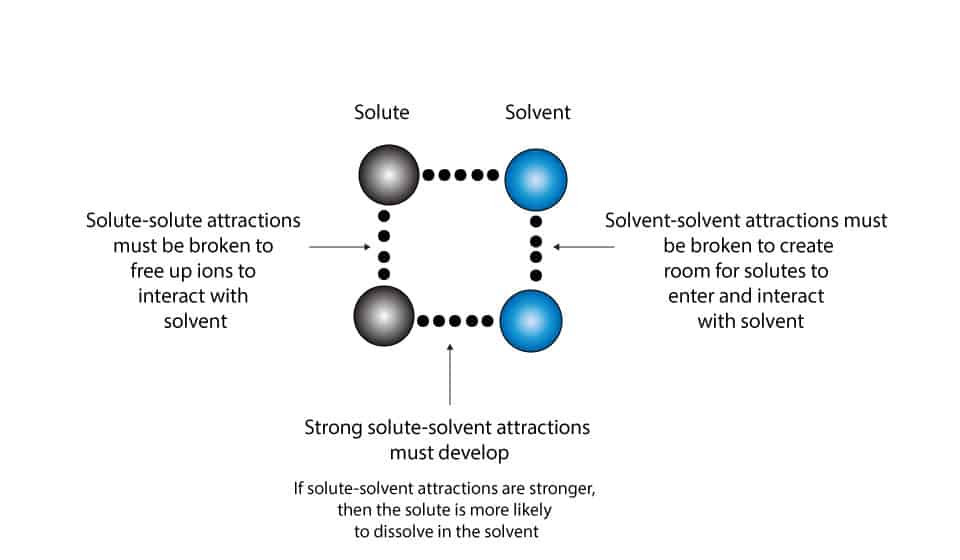
Solvents That Interact Weakly With The Stationary Phase . The interaction of a solvent molecule with an analyte depends on several forces: Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent like acetonitrile] makes it possible to separate and. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. In rplc, the stationary phase is nonpolar or weakly polar. Dispersion, dipole, hydrogen bonding and. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of.
from mungfali.com
With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. The interaction of a solvent molecule with an analyte depends on several forces: Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent like acetonitrile] makes it possible to separate and. Dispersion, dipole, hydrogen bonding and. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. In rplc, the stationary phase is nonpolar or weakly polar. Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis.
Solvent And Solute
Solvents That Interact Weakly With The Stationary Phase Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. Dispersion, dipole, hydrogen bonding and. In rplc, the stationary phase is nonpolar or weakly polar. Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent like acetonitrile] makes it possible to separate and. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. The interaction of a solvent molecule with an analyte depends on several forces: Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of.
From slideplayer.com
Thin Layer Chromatography ppt download Solvents That Interact Weakly With The Stationary Phase Dispersion, dipole, hydrogen bonding and. Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent like acetonitrile] makes it possible to separate and. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. In rplc, the stationary phase. Solvents That Interact Weakly With The Stationary Phase.
From www.scribd.com
Solute Solvent Interactions PPI BPII I PDF Intermolecular Force Solvents That Interact Weakly With The Stationary Phase Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent like. Solvents That Interact Weakly With The Stationary Phase.
From slideplayer.com
An Introduction to Chromatographic Separations ppt download Solvents That Interact Weakly With The Stationary Phase In rplc, the stationary phase is nonpolar or weakly polar. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. Dispersion, dipole, hydrogen bonding and. The main principle that allows chromatography. Solvents That Interact Weakly With The Stationary Phase.
From mungfali.com
Solvent And Solute Solvents That Interact Weakly With The Stationary Phase With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. In rplc, the stationary. Solvents That Interact Weakly With The Stationary Phase.
From www.slideserve.com
PPT Stationary phase Mobile phase PowerPoint Presentation, free Solvents That Interact Weakly With The Stationary Phase The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. In rplc, the stationary phase is nonpolar or weakly polar. Mcreynolds constants can. Solvents That Interact Weakly With The Stationary Phase.
From www.researchgate.net
1 Solvents available for Download Scientific Diagram Solvents That Interact Weakly With The Stationary Phase In rplc, the stationary phase is nonpolar or weakly polar. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. With a strong. Solvents That Interact Weakly With The Stationary Phase.
From courses.lumenlearning.com
The Dissolution Process Chemistry for Majors Solvents That Interact Weakly With The Stationary Phase With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. In rplc, the stationary phase is nonpolar or weakly polar. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. Learn. Solvents That Interact Weakly With The Stationary Phase.
From shaunmwilliams.com
Chapter 11 Presentation Solvents That Interact Weakly With The Stationary Phase With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. In rplc, the stationary phase is nonpolar or weakly polar. The. Solvents That Interact Weakly With The Stationary Phase.
From www.slideserve.com
PPT What Is HPLC? PowerPoint Presentation, free download ID4713396 Solvents That Interact Weakly With The Stationary Phase Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. Learn how to select the optimal solid phase extraction (spe) stationary phase to. Solvents That Interact Weakly With The Stationary Phase.
From www.slideserve.com
PPT PRINCIPLES OF CHEMISTRY II CHEM 1212 CHAPTER 12 PowerPoint Solvents That Interact Weakly With The Stationary Phase Dispersion, dipole, hydrogen bonding and. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. The interaction of a solvent molecule with an analyte depends on several forces: Mcreynolds constants can be used to estimate the ability of a stationary phase. Solvents That Interact Weakly With The Stationary Phase.
From theory.labster.com
Chromatography Labster Theory Solvents That Interact Weakly With The Stationary Phase The interaction of a solvent molecule with an analyte depends on several forces: Dispersion, dipole, hydrogen bonding and. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary. Solvents That Interact Weakly With The Stationary Phase.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID1406269 Solvents That Interact Weakly With The Stationary Phase Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent. Solvents That Interact Weakly With The Stationary Phase.
From www.waters.com
Beginner's Guide to Convergence Chromatography 2 Waters Solvents That Interact Weakly With The Stationary Phase Dispersion, dipole, hydrogen bonding and. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. With a strong polar adsorbent stationary phase like. Solvents That Interact Weakly With The Stationary Phase.
From www.chemistrysteps.com
The Role of Solvent in SN1, SN2, E1 and E2 Reactions Chemistry Steps Solvents That Interact Weakly With The Stationary Phase Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent like acetonitrile] makes it possible to separate and. In rplc, the stationary phase is nonpolar or weakly polar. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of.. Solvents That Interact Weakly With The Stationary Phase.
From www.researchgate.net
Stationary phase diagram for TASEP with intermolecular interactions Solvents That Interact Weakly With The Stationary Phase Dispersion, dipole, hydrogen bonding and. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. In rplc, the stationary phase is nonpolar or weakly polar. Adding some water [<20%] to. Solvents That Interact Weakly With The Stationary Phase.
From www.numerade.com
SOLVEDThe stationary phase and mobile phase interactions A wide Solvents That Interact Weakly With The Stationary Phase Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. The interaction of a solvent molecule with an analyte depends on several forces:. Solvents That Interact Weakly With The Stationary Phase.
From www.youtube.com
What are possible interaction between stationary phase & solute in RPLC Solvents That Interact Weakly With The Stationary Phase Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent like acetonitrile] makes it possible to separate and. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted. Solvents That Interact Weakly With The Stationary Phase.
From www.slideserve.com
PPT THIN LAYER CHROMATOGRAPHY PowerPoint Presentation, free download Solvents That Interact Weakly With The Stationary Phase Dispersion, dipole, hydrogen bonding and. The interaction of a solvent molecule with an analyte depends on several forces: Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. In rplc, the stationary phase is nonpolar or weakly polar. The main principle that allows chromatography to separate components of a mixture. Solvents That Interact Weakly With The Stationary Phase.
From chempedia.info
Normalphase chromatography retention Big Chemical Encyclopedia Solvents That Interact Weakly With The Stationary Phase The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the. Solvents That Interact Weakly With The Stationary Phase.
From www.slideserve.com
PPT LIQUID CHROMATOGRAPHY PowerPoint Presentation, free download ID Solvents That Interact Weakly With The Stationary Phase Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by. Solvents That Interact Weakly With The Stationary Phase.
From www.chegg.com
Solved 1. What is the definition of weak stationarity? 2. Solvents That Interact Weakly With The Stationary Phase Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. The interaction of a. Solvents That Interact Weakly With The Stationary Phase.
From www.researchgate.net
Types of interactions with stationary phase characterized by McReynolds Solvents That Interact Weakly With The Stationary Phase Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent like acetonitrile] makes it possible to separate and. Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes.. Solvents That Interact Weakly With The Stationary Phase.
From www.researchgate.net
Schematic illustration of the neutral particle interactions in solvents Solvents That Interact Weakly With The Stationary Phase Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent like acetonitrile] makes it possible to separate and. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. Dispersion, dipole, hydrogen bonding and. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as. Solvents That Interact Weakly With The Stationary Phase.
From chemistry-europe.onlinelibrary.wiley.com
The Solvent Effect on Weak Interactions in Supramolecular Polymers Solvents That Interact Weakly With The Stationary Phase The interaction of a solvent molecule with an analyte depends on several forces: Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. In rplc, the stationary phase is nonpolar or weakly polar. Adding some water [<20%] to the organic mobile phase [typically an aprotic solvent like acetonitrile] makes it possible. Solvents That Interact Weakly With The Stationary Phase.
From www.youtube.com
Solute Solvent Interactions part I Types of Solvents and their Solvents That Interact Weakly With The Stationary Phase The interaction of a solvent molecule with an analyte depends on several forces: With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various. Solvents That Interact Weakly With The Stationary Phase.
From www.goldbio.com
How Column Chromatography Works to Separate Proteins GoldBio Solvents That Interact Weakly With The Stationary Phase The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. In rplc, the stationary phase is nonpolar or weakly polar. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. Dispersion, dipole, hydrogen bonding and. With a strong polar adsorbent. Solvents That Interact Weakly With The Stationary Phase.
From www.researchgate.net
Illustrations of (a) solute solvation which is divided into cavity Solvents That Interact Weakly With The Stationary Phase Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as. Solvents That Interact Weakly With The Stationary Phase.
From solvedlib.com
In Thin layer chromatography, the stationary phase is… SolvedLib Solvents That Interact Weakly With The Stationary Phase Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. Dispersion, dipole, hydrogen bonding and. The interaction of a solvent molecule. Solvents That Interact Weakly With The Stationary Phase.
From www.slideserve.com
PPT Solvation Models PowerPoint Presentation, free download ID927911 Solvents That Interact Weakly With The Stationary Phase Dispersion, dipole, hydrogen bonding and. In rplc, the stationary phase is nonpolar or weakly polar. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. Adding some water [<20%] to the. Solvents That Interact Weakly With The Stationary Phase.
From www.researchgate.net
Scheme 9 The schematic representation of solutesolvent interaction Solvents That Interact Weakly With The Stationary Phase Both compounds are able to hydrogen bond to the polar stationary phase (figure 2.19a+b), so are more strongly attracted to the stationary phase than ethylbenzene, which interacts. Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. The main principle that allows chromatography to separate components of a mixture is that. Solvents That Interact Weakly With The Stationary Phase.
From www.chegg.com
Solved Question 5 Solvents that interact WEAKLY with the Solvents That Interact Weakly With The Stationary Phase Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. The main principle that allows chromatography to separate components of a mixture is that components will spend different amounts of. The interaction of a solvent molecule with an analyte depends on several forces: Dispersion, dipole, hydrogen bonding and. In rplc,. Solvents That Interact Weakly With The Stationary Phase.
From www.slideserve.com
PPT A practical approach to metabolomics PowerPoint Presentation Solvents That Interact Weakly With The Stationary Phase Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. Dispersion, dipole, hydrogen bonding and. In rplc, the stationary phase is. Solvents That Interact Weakly With The Stationary Phase.
From www.numerade.com
SOLVED Solvents that interact WEAKLY with the stationary phase are Solvents That Interact Weakly With The Stationary Phase Dispersion, dipole, hydrogen bonding and. With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. Both compounds are able to hydrogen bond. Solvents That Interact Weakly With The Stationary Phase.
From www.mdpi.com
Materials Free FullText Stationary Phases for Green Liquid Solvents That Interact Weakly With The Stationary Phase With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. Mcreynolds constants can be used to estimate the ability of a stationary phase to separate analytes of various compound classes. The interaction of a solvent molecule with an analyte depends on. Solvents That Interact Weakly With The Stationary Phase.
From www.slideserve.com
PPT What Is HPLC? PowerPoint Presentation, free download ID4713396 Solvents That Interact Weakly With The Stationary Phase With a strong polar adsorbent stationary phase like alumina, a polar solvent used as the mobile phase will be adsorbed by the stationary phase, which may displace molecules of. Learn how to select the optimal solid phase extraction (spe) stationary phase to cleanup your sample for chromatographic analysis. Dispersion, dipole, hydrogen bonding and. Adding some water [<20%] to the organic. Solvents That Interact Weakly With The Stationary Phase.