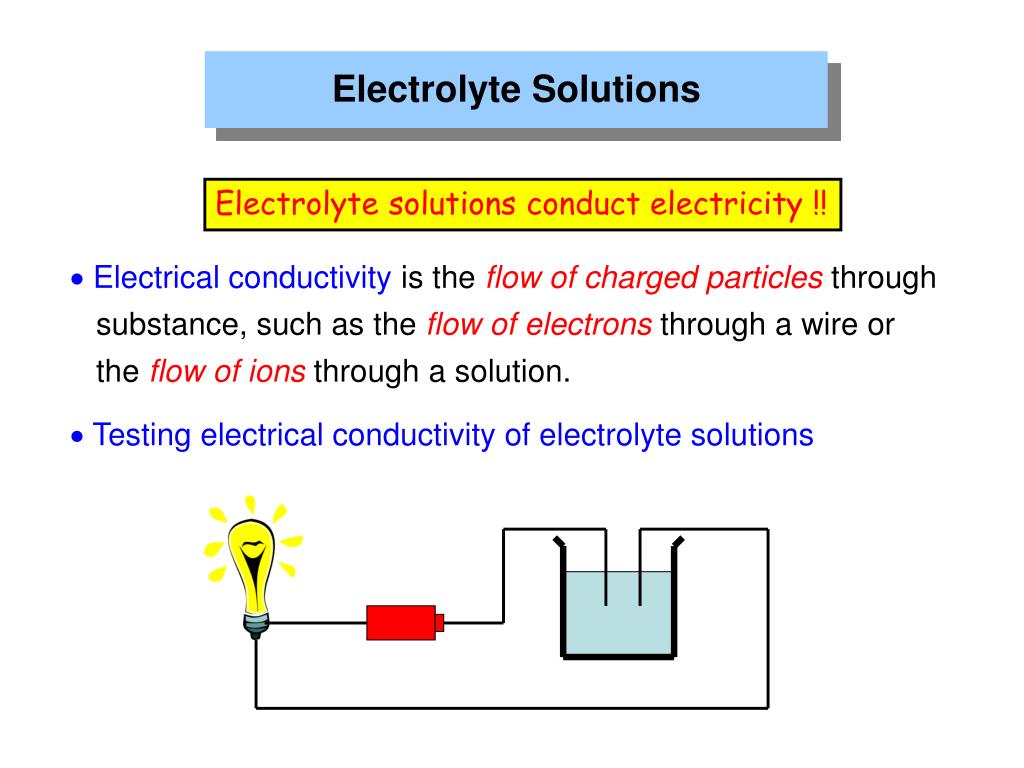
Concentrated Electrolyte Solutions Examples . Examples of electrolyte replacement solutions. Control of concentrated electrolyte solutions. Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). For example, \ (\ce {nh4oh}\). Some neutral molecules are present in their solutions. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. A common example of an electrolyte is ordinary salt, sodium chloride. Small fractions of weak electrolytes' molecules ionize when dissolve in water. For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. The larger the concentration of ions, the better the solutions. With more and more dilute solutions, you will get less chlorine and more. Below are two of the solutions commonly used to replace electrolytes.
from www.slideserve.com
Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. Examples of electrolyte replacement solutions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). For example, \ (\ce {nh4oh}\). Control of concentrated electrolyte solutions. A common example of an electrolyte is ordinary salt, sodium chloride. Some neutral molecules are present in their solutions. Below are two of the solutions commonly used to replace electrolytes. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. The larger the concentration of ions, the better the solutions.
PPT Chemistry 101 Chap. 4 PowerPoint Presentation, free download
Concentrated Electrolyte Solutions Examples An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Below are two of the solutions commonly used to replace electrolytes. With more and more dilute solutions, you will get less chlorine and more. For example, \ (\ce {nh4oh}\). For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. The larger the concentration of ions, the better the solutions. Some neutral molecules are present in their solutions. Control of concentrated electrolyte solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. A common example of an electrolyte is ordinary salt, sodium chloride. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. Examples of electrolyte replacement solutions.
From www.youtube.com
Lab 9 Solutions, Electrolytes and Concentrations YouTube Concentrated Electrolyte Solutions Examples Examples of electrolyte replacement solutions. A common example of an electrolyte is ordinary salt, sodium chloride. Control of concentrated electrolyte solutions. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. Below are two of the solutions commonly used to replace electrolytes. Intravenous fluids (iv fluids), also known as intravenous solutions,. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT Chemistry 101 Chap. 4 PowerPoint Presentation, free download Concentrated Electrolyte Solutions Examples The larger the concentration of ions, the better the solutions. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. With more and more dilute solutions, you will get less chlorine and more. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Examples of electrolyte replacement solutions. For example,. Concentrated Electrolyte Solutions Examples.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Concentrated Electrolyte Solutions Examples Examples of electrolyte replacement solutions. With more and more dilute solutions, you will get less chlorine and more. Control of concentrated electrolyte solutions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Small fractions of weak electrolytes' molecules ionize when dissolve in water. For example, if you have a concentrated solution of sodium. Concentrated Electrolyte Solutions Examples.
From scottscience.weebly.com
Unit 11 Solutions Mr. Scott's Online Classroom Concentrated Electrolyte Solutions Examples For example, \ (\ce {nh4oh}\). Some neutral molecules are present in their solutions. The larger the concentration of ions, the better the solutions. For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. Examples of electrolyte replacement solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in water. A common. Concentrated Electrolyte Solutions Examples.
From www.shutterstock.com
전해질 및 비전해질 용액의 인포그래픽 다이어그램은 스톡 일러스트 1076670605 Shutterstock Concentrated Electrolyte Solutions Examples Some neutral molecules are present in their solutions. Below are two of the solutions commonly used to replace electrolytes. Control of concentrated electrolyte solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in water. For example, \ (\ce {nh4oh}\). For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. An. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT Chapter 10 Properties of Solutions PowerPoint Presentation ID Concentrated Electrolyte Solutions Examples With more and more dilute solutions, you will get less chlorine and more. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. The larger the concentration of ions, the better the solutions. Examples of electrolyte replacement solutions. For example, \ (\ce {nh4oh}\). Small fractions of weak electrolytes' molecules ionize when. Concentrated Electrolyte Solutions Examples.
From studymind.co.uk
Electrolysis of Aqueous Solutions (GCSE Chemistry) Study Mind Concentrated Electrolyte Solutions Examples Examples of electrolyte replacement solutions. With more and more dilute solutions, you will get less chlorine and more. The larger the concentration of ions, the better the solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in water. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). For example, if you have. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT Solutions Review Pt 2 PowerPoint Presentation, free download ID Concentrated Electrolyte Solutions Examples An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Below are two of the solutions commonly used to replace electrolytes. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. A common example of an electrolyte is ordinary salt, sodium chloride. For example, if. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT Chemistry 101 Chap. 4 PowerPoint Presentation, free download Concentrated Electrolyte Solutions Examples With more and more dilute solutions, you will get less chlorine and more. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. A common example of an electrolyte is ordinary salt, sodium chloride. For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at. Concentrated Electrolyte Solutions Examples.
From www.slideshare.net
Chapter 16 solutions Concentrated Electrolyte Solutions Examples A common example of an electrolyte is ordinary salt, sodium chloride. Examples of electrolyte replacement solutions. For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Control of concentrated electrolyte solutions. In these examples, the. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT Electrical Conductivity PowerPoint Presentation, free download Concentrated Electrolyte Solutions Examples Examples of electrolyte replacement solutions. Below are two of the solutions commonly used to replace electrolytes. Some neutral molecules are present in their solutions. A common example of an electrolyte is ordinary salt, sodium chloride. For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. Small fractions of weak electrolytes' molecules. Concentrated Electrolyte Solutions Examples.
From www.youtube.com
EMF of Electrolyte Concentration Cell Reversible to Cation with Concentrated Electrolyte Solutions Examples With more and more dilute solutions, you will get less chlorine and more. Examples of electrolyte replacement solutions. Control of concentrated electrolyte solutions. The larger the concentration of ions, the better the solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in water. A common example of an electrolyte is ordinary salt, sodium chloride. For example, \ (\ce {nh4oh}\).. Concentrated Electrolyte Solutions Examples.
From www.youtube.com
4.1 Solutions, Aqueous Solutions, Electrolytes & Concentrations YouTube Concentrated Electrolyte Solutions Examples Examples of electrolyte replacement solutions. For example, \ (\ce {nh4oh}\). Small fractions of weak electrolytes' molecules ionize when dissolve in water. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. Below are two of. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID748165 Concentrated Electrolyte Solutions Examples A common example of an electrolyte is ordinary salt, sodium chloride. For example, \ (\ce {nh4oh}\). Some neutral molecules are present in their solutions. Examples of electrolyte replacement solutions. The larger the concentration of ions, the better the solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Intravenous fluids (iv fluids), also known as intravenous solutions, are. Concentrated Electrolyte Solutions Examples.
From revisechemistry.uk
Electrolysis AQA C4 revisechemistry.uk Concentrated Electrolyte Solutions Examples Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. Some neutral molecules are present in their solutions. The larger the concentration of ions, the better the solutions. For example, \ (\ce {nh4oh}\). Control of concentrated electrolyte solutions. For example, if you have a concentrated solution of sodium chloride, you will. Concentrated Electrolyte Solutions Examples.
From www.onlinemathlearning.com
Chemical Reactions IGCSE Chemistry (solutions, examples, worksheets Concentrated Electrolyte Solutions Examples Some neutral molecules are present in their solutions. Control of concentrated electrolyte solutions. Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. With more and more dilute solutions, you will get less chlorine and more. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to. Concentrated Electrolyte Solutions Examples.
From study.com
Electrolysis of Aqueous Solutions Lesson Concentrated Electrolyte Solutions Examples Small fractions of weak electrolytes' molecules ionize when dissolve in water. For example, \ (\ce {nh4oh}\). In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. With more and more dilute solutions, you will get less chlorine and more. An electrolyte solution conducts electricity because of the movement of ions in. Concentrated Electrolyte Solutions Examples.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Concentrated Electrolyte Solutions Examples For example, \ (\ce {nh4oh}\). Control of concentrated electrolyte solutions. A common example of an electrolyte is ordinary salt, sodium chloride. Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. Below are two of the solutions commonly used to replace electrolytes. An electrolyte solution conducts electricity because of the movement. Concentrated Electrolyte Solutions Examples.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Concentrated Electrolyte Solutions Examples For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. A common example of an electrolyte is ordinary salt, sodium chloride. Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. Small fractions of weak electrolytes' molecules ionize when dissolve in water.. Concentrated Electrolyte Solutions Examples.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary Concentrated Electrolyte Solutions Examples With more and more dilute solutions, you will get less chlorine and more. Control of concentrated electrolyte solutions. Examples of electrolyte replacement solutions. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Below are two of the solutions commonly. Concentrated Electrolyte Solutions Examples.
From www.researchgate.net
Multiple functions of the concentrated electrolyte in lithium metal Concentrated Electrolyte Solutions Examples For example, \ (\ce {nh4oh}\). Control of concentrated electrolyte solutions. With more and more dilute solutions, you will get less chlorine and more. Below are two of the solutions commonly used to replace electrolytes. Small fractions of weak electrolytes' molecules ionize when dissolve in water. A common example of an electrolyte is ordinary salt, sodium chloride. Intravenous fluids (iv fluids),. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT SOLUTIONS OF ELECTROLYTES PowerPoint Presentation, free download Concentrated Electrolyte Solutions Examples In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. The larger the concentration of ions, the better the solutions. With more and more dilute solutions, you will get less chlorine and more. Some neutral molecules are present in their solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID181760 Concentrated Electrolyte Solutions Examples Below are two of the solutions commonly used to replace electrolytes. Examples of electrolyte replacement solutions. Control of concentrated electrolyte solutions. The larger the concentration of ions, the better the solutions. Some neutral molecules are present in their solutions. With more and more dilute solutions, you will get less chlorine and more. In these examples, the authors attribute the improved. Concentrated Electrolyte Solutions Examples.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Concentrated Electrolyte Solutions Examples Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. Small fractions of weak electrolytes' molecules ionize when dissolve in water. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. For example, if you have a concentrated solution of sodium chloride,. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID6585303 Concentrated Electrolyte Solutions Examples The larger the concentration of ions, the better the solutions. Some neutral molecules are present in their solutions. For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. Below are two of the solutions commonly used to replace electrolytes. In these examples, the authors attribute the improved lithium metal deposition (and. Concentrated Electrolyte Solutions Examples.
From www.researchgate.net
Schematic illustration of three types of electrolytes. Download Concentrated Electrolyte Solutions Examples Some neutral molecules are present in their solutions. Below are two of the solutions commonly used to replace electrolytes. Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. A common example of an electrolyte is ordinary salt, sodium chloride. For example, if you have a concentrated solution of sodium chloride,. Concentrated Electrolyte Solutions Examples.
From exoabfmik.blob.core.windows.net
PreLab Quiz Solutions Electrolytes And Concentration at Manuel Concentrated Electrolyte Solutions Examples Small fractions of weak electrolytes' molecules ionize when dissolve in water. Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. Some neutral molecules are present in their solutions. In these examples,. Concentrated Electrolyte Solutions Examples.
From www.animalia-life.club
Electrolyte Chemistry Concentrated Electrolyte Solutions Examples For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. Examples of electrolyte replacement solutions. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Control of concentrated electrolyte solutions. Below. Concentrated Electrolyte Solutions Examples.
From courses.lumenlearning.com
Electrolysis Chemistry Concentrated Electrolyte Solutions Examples For example, \ (\ce {nh4oh}\). With more and more dilute solutions, you will get less chlorine and more. A common example of an electrolyte is ordinary salt, sodium chloride. Examples of electrolyte replacement solutions. Control of concentrated electrolyte solutions. The larger the concentration of ions, the better the solutions. Some neutral molecules are present in their solutions. For example, if. Concentrated Electrolyte Solutions Examples.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps Concentrated Electrolyte Solutions Examples A common example of an electrolyte is ordinary salt, sodium chloride. Examples of electrolyte replacement solutions. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. Some neutral molecules are present in their solutions. With more and more dilute solutions, you will get less chlorine and more. Below are two of. Concentrated Electrolyte Solutions Examples.
From slidetodoc.com
Lecture 9 Electrical conductivity of electrolytes solutions Prepared Concentrated Electrolyte Solutions Examples An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Some neutral molecules are present in their solutions. Control of concentrated electrolyte solutions. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. A common example of an electrolyte is ordinary salt, sodium chloride. Small. Concentrated Electrolyte Solutions Examples.
From exokypmcv.blob.core.windows.net
Homemade Oral Electrolyte Solution at Dorothy Kelley blog Concentrated Electrolyte Solutions Examples Intravenous fluids (iv fluids), also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Control of concentrated electrolyte solutions. For example, \ (\ce {nh4oh}\). Some neutral molecules are present in their solutions. With more and more dilute solutions, you. Concentrated Electrolyte Solutions Examples.
From studylib.net
Control of Concentrated Electrolyte Solutions Concentrated Electrolyte Solutions Examples Small fractions of weak electrolytes' molecules ionize when dissolve in water. For example, \ (\ce {nh4oh}\). An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Examples of electrolyte replacement solutions. Some neutral molecules are present in their solutions. Below are two of the solutions commonly used to replace electrolytes. In these examples, the. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT 24.6 The conductivities of electrolyte solutions PowerPoint Concentrated Electrolyte Solutions Examples The larger the concentration of ions, the better the solutions. With more and more dilute solutions, you will get less chlorine and more. For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at the anode. Some neutral molecules are present in their solutions. Examples of electrolyte replacement solutions. Control of concentrated electrolyte solutions.. Concentrated Electrolyte Solutions Examples.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3154052 Concentrated Electrolyte Solutions Examples Below are two of the solutions commonly used to replace electrolytes. In these examples, the authors attribute the improved lithium metal deposition (and dissolution) behavior to the changes in the. With more and more dilute solutions, you will get less chlorine and more. For example, if you have a concentrated solution of sodium chloride, you will get mainly chlorine at. Concentrated Electrolyte Solutions Examples.