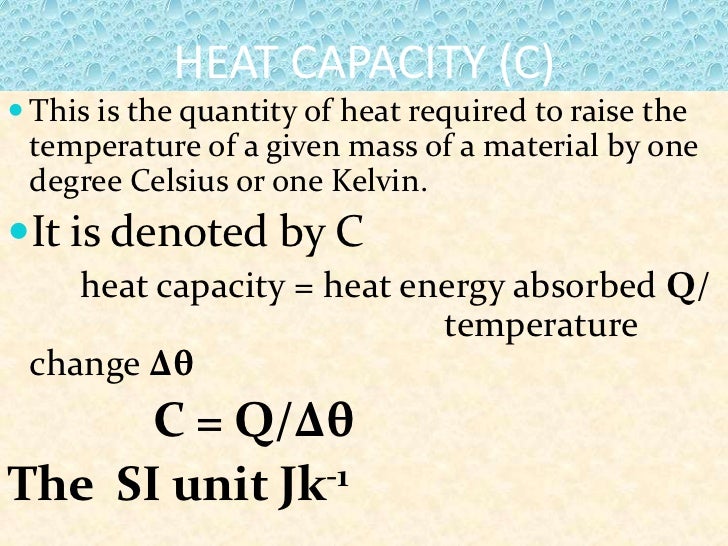
Define Heat Quantity . The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Define heat capacity and specific heat capacity and differentiate between the two terms; Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Deduce which substance will have. It is usually expressed as calories per degree in. Its si unit is j k −1. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,.
from www.slideshare.net
Heat capacity, ratio of heat absorbed by a material to the temperature change. Deduce which substance will have. It plays a crucial role in understanding. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. It is usually expressed as calories per degree in. Its si unit is j k −1.
Quantity of heat
Define Heat Quantity It plays a crucial role in understanding. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. It plays a crucial role in understanding. Define heat capacity and specific heat capacity and differentiate between the two terms; The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Its si unit is j k −1. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. It is usually expressed as calories per degree in. Deduce which substance will have.
From slideshare.net
Heat Capacity Define Heat Quantity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. It plays a crucial role in understanding. Deduce which substance. Define Heat Quantity.
From www.slideserve.com
PPT Chapter 15 TEMPERATURE, HEAT AND EXPANSION PowerPoint Define Heat Quantity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Deduce which substance will have. The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles.. Define Heat Quantity.
From www.slideserve.com
PPT Fundamentals of Heat Transfer PowerPoint Presentation, free Define Heat Quantity Deduce which substance will have. The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material. Define Heat Quantity.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources Define Heat Quantity Its si unit is j k −1. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. It plays a crucial role in understanding. It is usually expressed as calories per degree in. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius.. Define Heat Quantity.
From studylib.net
1.3 Specific Heat Capacity Define Heat Quantity It plays a crucial role in understanding. Its si unit is j k −1. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. The heat. Define Heat Quantity.
From dxoqtwvpm.blob.core.windows.net
What Is Heat Capacity In Materials at James Eng blog Define Heat Quantity Define heat capacity and specific heat capacity and differentiate between the two terms; Deduce which substance will have. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. The molar heat capacity is determined by dividing the heat capacity by the sum. Define Heat Quantity.
From www.slideshare.net
Heat Capacity Define Heat Quantity Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Define heat capacity and specific heat capacity and differentiate between the two. Define Heat Quantity.
From gamesmartz.com
Heat Capacity Definition Easy to Understand Define Heat Quantity Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. The heat capacity of a body is the quantity of heat required to raise. Define Heat Quantity.
From www.slideserve.com
PPT Chapter 17 The first law of thermodynamics PowerPoint Define Heat Quantity Define heat capacity and specific heat capacity and differentiate between the two terms; Heat capacity, ratio of heat absorbed by a material to the temperature change. Its si unit is j k −1. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Thermal energy, in turn, is the kinetic energy of vibrating and colliding.. Define Heat Quantity.
From www.slideshare.net
Quantity of heat Define Heat Quantity The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is usually expressed as calories per degree in. It plays a crucial role in understanding.. Define Heat Quantity.
From haikudeck.com
Quantity Of Heat. by Boniface Thuranira Define Heat Quantity Deduce which substance will have. The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. It plays a crucial. Define Heat Quantity.
From jahamattbrown.blogspot.com
Specific Heat Capacity Definition Matt Brown Define Heat Quantity The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. Its si unit is j k −1. It plays a crucial role in understanding. Deduce which substance will have. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity, also known. Define Heat Quantity.
From www.chegg.com
Solved Question 11 5 pts Define heat capacity. the quantity Define Heat Quantity Define heat capacity and specific heat capacity and differentiate between the two terms; It plays a crucial role in understanding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Deduce which substance will have. Heat capacity, also known as thermal capacity, is a physical. Define Heat Quantity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Define Heat Quantity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. Its si. Define Heat Quantity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Define Heat Quantity Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. Define. Define Heat Quantity.
From www.slideshare.net
Quantity of heat Define Heat Quantity Its si unit is j k −1. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1. Define Heat Quantity.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems Define Heat Quantity Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. The heat capacity of a body is the quantity of heat required to raise its. Define Heat Quantity.
From www.downwindkennels.com
Specific Heat Capacity Define Heat Quantity Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. It is usually expressed as calories per degree in. Its si unit is j k. Define Heat Quantity.
From printablelibagnames.z13.web.core.windows.net
Calculate The Quantity Of Heat Define Heat Quantity The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. It is usually expressed as calories per degree in. Deduce which substance will have. The heat capacity (c) of a body of matter is the quantity of. Define Heat Quantity.
From www.slideshare.net
Quantity of heat Define Heat Quantity Its si unit is j k −1. It plays a crucial role in understanding. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. Heat is the thermal energy transfer between systems or. Define Heat Quantity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Define Heat Quantity The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the. Define Heat Quantity.
From www.slideserve.com
PPT Properties of Materials PowerPoint Presentation, free download Define Heat Quantity Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. It is usually expressed as calories per degree in. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or. Define Heat Quantity.
From www.slideserve.com
PPT Advance Chemical Engineering Thermodynamics PowerPoint Define Heat Quantity Deduce which substance will have. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. Heat capacity, also known as thermal capacity, is a physical property. Define Heat Quantity.
From slideplayer.com
Chapter 6 Thermochemistry. Copyright © Cengage Learning. All rights Define Heat Quantity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. It plays a crucial role in understanding. Specific heat. Define Heat Quantity.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula Define Heat Quantity The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Deduce which substance will have. It plays a crucial role in understanding. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Specific heat is defined as the amount of heat required to raise the temperature of a unit. Define Heat Quantity.
From ispitna.blogspot.com
Q Formula Chemistry Ispitna Define Heat Quantity Its si unit is j k −1. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Deduce which substance will have. The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. It plays a crucial role in understanding. Define heat capacity and specific heat capacity and differentiate between the. Define Heat Quantity.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download Define Heat Quantity It plays a crucial role in understanding. The molar heat capacity is determined by dividing the heat capacity by the sum of substances in moles. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat capacity, also known. Define Heat Quantity.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity Define Heat Quantity The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. Specific heat is defined as the amount of. Define Heat Quantity.
From www.youtube.com
Lecture 14 What is Heat Capacity? YouTube Define Heat Quantity It is usually expressed as calories per degree in. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. Heat capacity, ratio of heat absorbed by a material to the temperature change. Deduce. Define Heat Quantity.
From www.youtube.com
Specific heat capacity class 11 physics YouTube Define Heat Quantity Its si unit is j k −1. It is usually expressed as calories per degree in. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. The heat capacity of a body is the quantity of heat required. Define Heat Quantity.
From klargdopl.blob.core.windows.net
Short Definition Of Heat at Robert Petrin blog Define Heat Quantity It plays a crucial role in understanding. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The molar heat capacity is determined by dividing the. Define Heat Quantity.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free Define Heat Quantity It plays a crucial role in understanding. Heat capacity, also known as thermal capacity, is a physical property of matter defined as the amount of heat needed to cause a unit change in temperature in a given mass of material. It is usually expressed as calories per degree in. Its si unit is j k −1. The heat capacity of. Define Heat Quantity.
From www.slideserve.com
PPT Temperature and Heat PowerPoint Presentation ID3080166 Define Heat Quantity Heat capacity, ratio of heat absorbed by a material to the temperature change. Thermal energy, in turn, is the kinetic energy of vibrating and colliding. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. It is usually. Define Heat Quantity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Define Heat Quantity The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity, ratio of heat absorbed by a material to the temperature change. Deduce which substance will have. It plays a crucial role in understanding. It is usually expressed as calories per degree in. Define heat capacity and specific heat capacity. Define Heat Quantity.
From www.youtube.com
Specific heat capacity YouTube Define Heat Quantity Heat is the thermal energy transfer between systems or bodies due to a temperature difference. It plays a crucial role in understanding. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. Heat capacity, also known as thermal. Define Heat Quantity.