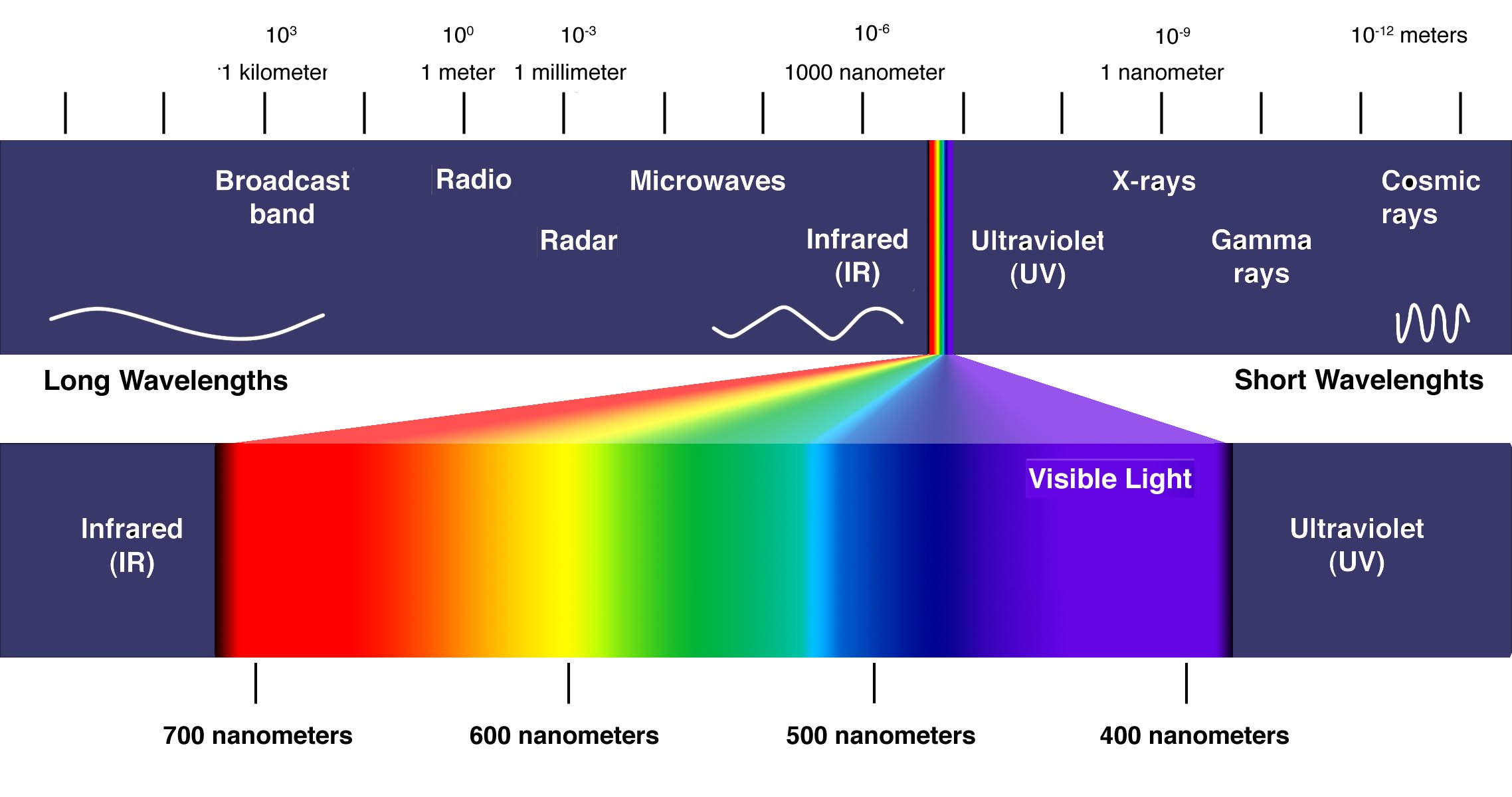
Emission Spectra Visible Light . the emission spectra of various atoms. Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The figure below shows the atomic emission spectrum of hydrogen. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains.
from newgradoptometry.com
this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. the emission spectra of various atoms. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The figure below shows the atomic emission spectrum of hydrogen.
Understanding Acuvue Contacts and Ultraviolet Light
Emission Spectra Visible Light an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. the emission spectra of various atoms. The figure below shows the atomic emission spectrum of hydrogen. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line.
From factmyth.com
Visible Light is Radiation Fact or Myth? Emission Spectra Visible Light Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the. Emission Spectra Visible Light.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Emission Spectra Visible Light the emission spectra of various atoms. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. The figure below shows the atomic emission spectrum of hydrogen. the emission spectrum (or line. Emission Spectra Visible Light.
From www.freepik.com
Premium Vector Visible light diagram. Color spectrum Emission Spectra Visible Light this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. an atomic. Emission Spectra Visible Light.
From imagine.gsfc.nasa.gov
Spectra Introduction Emission Spectra Visible Light The figure below shows the atomic emission spectrum of hydrogen. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. the emission spectra of various atoms. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of. Emission Spectra Visible Light.
From www.freepik.com
Premium Vector Spectrum wavelength Visible spectrum color range Emission Spectra Visible Light the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. for higher physics, revise emission or absorption of certain frequencies of light from the. Emission Spectra Visible Light.
From www.gatan.com
Complete understanding of light emission with nanoscale spatial Emission Spectra Visible Light this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The figure below shows the atomic emission spectrum of hydrogen. the emission spectra. Emission Spectra Visible Light.
From sciencenotes.org
Visible Light Spectrum Wavelengths and Colors Emission Spectra Visible Light an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The figure below shows the atomic emission spectrum of hydrogen. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. When hydrogen gas. Emission Spectra Visible Light.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Emission Spectra Visible Light this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the. Emission Spectra Visible Light.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Emission Spectra Visible Light the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. When hydrogen gas is placed into a tube and electric current passed through it, the. Emission Spectra Visible Light.
From mungfali.com
Visible Spectrum Color Wheel Emission Spectra Visible Light the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. The figure below shows the atomic emission spectrum of hydrogen. the emission spectra. Emission Spectra Visible Light.
From www.thoughtco.com
Visible Light Spectrum Overview and Chart Emission Spectra Visible Light an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. the emission spectra of various atoms. Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. the emission spectrum (or line spectrum). Emission Spectra Visible Light.
From www.researchgate.net
Basics of Fluorescence and FRET. ( a ) Visible light spectrum Emission Spectra Visible Light for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how. Emission Spectra Visible Light.
From stock.adobe.com
Continuous spectrum, an emission spectrum that consists of continuum of Emission Spectra Visible Light for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. an. Emission Spectra Visible Light.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Emission Spectra Visible Light the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. an atomic emission spectrum is the pattern of lines formed when. Emission Spectra Visible Light.
From printabletarareartn.z22.web.core.windows.net
Picture Of Visible Light Spectrum Emission Spectra Visible Light the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When hydrogen gas is placed. Emission Spectra Visible Light.
From www.dreamstime.com
Visible Light Spectrum Diagram Stock Illustration Illustration of Emission Spectra Visible Light Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. the emission. Emission Spectra Visible Light.
From www.alamy.com
Spectrum And Visible Light Stock Photo Alamy Emission Spectra Visible Light the emission spectra of various atoms. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of. Emission Spectra Visible Light.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Visible Light this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. the. Emission Spectra Visible Light.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Emission Spectra Visible Light an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. for higher physics, revise emission or absorption of certain frequencies of light from. Emission Spectra Visible Light.
From www.alamy.com
Visible color spectrum. Sunlight wavelength and increasing frequency Emission Spectra Visible Light the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The figure below shows the atomic emission spectrum of hydrogen. Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. for higher physics, revise. Emission Spectra Visible Light.
From www.researchgate.net
Emission spectra of different light sources (a) incandescent tungsten Emission Spectra Visible Light for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Examples of. Emission Spectra Visible Light.
From newgradoptometry.com
Understanding Acuvue Contacts and Ultraviolet Light Emission Spectra Visible Light Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. an atomic. Emission Spectra Visible Light.
From animalia-life.club
Spectrum Visible Light Prism Emission Spectra Visible Light this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. the emission spectra of various atoms. Examples of a continuous, an emission and an. Emission Spectra Visible Light.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Emission Spectra Visible Light this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. the emission spectra of various atoms. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how. Emission Spectra Visible Light.
From www.vecteezy.com
Visible light spectrum. Optical light wavelength. Emission Spectra Visible Light Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to. Emission Spectra Visible Light.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Emission Spectra Visible Light an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. the emission spectra of various atoms. Examples of a continuous, an emission and. Emission Spectra Visible Light.
From users.highland.edu
Atomic Spectra and Models of the Atom Emission Spectra Visible Light The figure below shows the atomic emission spectrum of hydrogen. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. the emission spectra of various atoms. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic. Emission Spectra Visible Light.
From www.researchgate.net
Emission spectra of LED light sources. Download Scientific Diagram Emission Spectra Visible Light the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. this experiment targets the visible region of this spectrum specifically, because we can. Emission Spectra Visible Light.
From jpl.nasa.gov
Educator Guide Math of the Expanding Universe NASA/JPL Edu Emission Spectra Visible Light an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Examples of a continuous, an emission and an absorption spectrum with. Emission Spectra Visible Light.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectra Visible Light Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The figure below shows the atomic emission spectrum of hydrogen. this experiment targets. Emission Spectra Visible Light.
From ecampusontario.pressbooks.pub
8.2 Atomic Spectra General Chemistry for GeeGees Emission Spectra Visible Light an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. for higher physics, revise. Emission Spectra Visible Light.
From sunwindsolar.com
Solar Radiation Spectrum • SunWind Solar Emission Spectra Visible Light When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The figure below shows the atomic emission spectrum of hydrogen. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. . Emission Spectra Visible Light.
From www.slideserve.com
PPT Properties of Light PowerPoint Presentation, free download ID Emission Spectra Visible Light for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. this experiment targets the visible region of this spectrum specifically, because we can see electromagnetic radiation with. Examples of a continuous, an emission and an absorption spectrum with diagrams illustrating how the spectra were obtained. the emission spectra of. Emission Spectra Visible Light.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Visible Light When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. this experiment targets the visible region of this spectrum specifically,. Emission Spectra Visible Light.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectra Visible Light for higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it. Emission Spectra Visible Light.