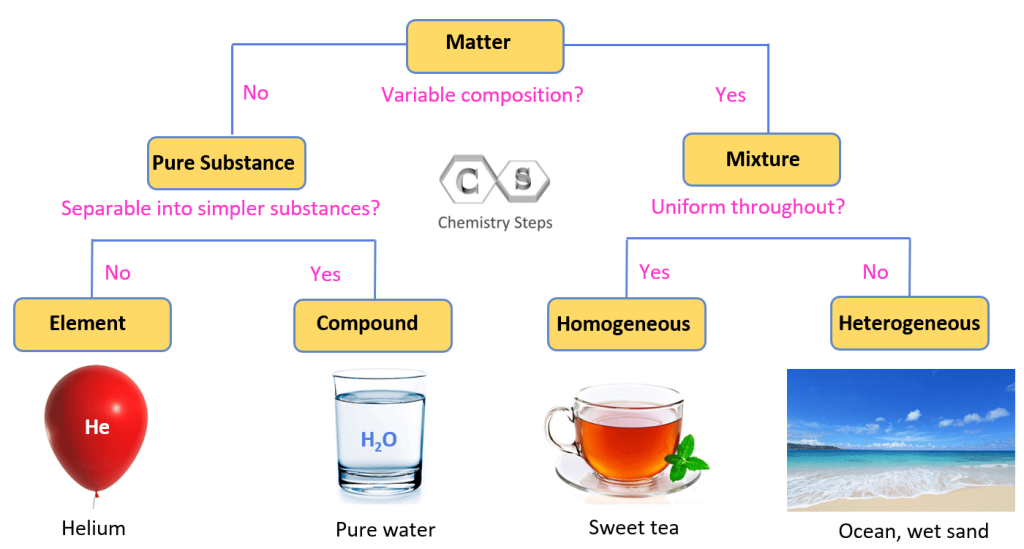
Homogeneous Mixture Solid Liquid And Gas . It is uniform in composition throughout. A homogeneous mixture is one that contains the same proportions of its. A homogeneous mixture appears uniform, regardless of where you sample it. describe the solid, liquid and gas phases. — the components of a mixture retain their own properties and can be solid, liquid or gas. — many common liquids are homogeneous mixtures. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — a mixture is formed by combining two or more materials. There is only one phase of matter observed in a homogeneous mixture. No matter where you sample the. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. Explain the difference between an element and. Examples include steel, the gases in a scuba tank. Explain the difference between a pure substance and a mixture. Examples include dishwashing liquid, shampoo, vinegar, wine,.
from general.chemistrysteps.com
— a mixture is formed by combining two or more materials. — there is no partitional difference in the combination. Examples include dishwashing liquid, shampoo, vinegar, wine,. It is uniform in composition throughout. No matter where you sample the. A homogeneous mixture appears uniform, regardless of where you sample it. — the components of a mixture retain their own properties and can be solid, liquid or gas. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. There is only one phase of matter observed in a homogeneous mixture. A homogeneous mixture is one that contains the same proportions of its.
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps
Homogeneous Mixture Solid Liquid And Gas A homogeneous mixture is one that contains the same proportions of its. Explain the difference between a pure substance and a mixture. It is uniform in composition throughout. A homogeneous mixture is one that contains the same proportions of its. Examples include dishwashing liquid, shampoo, vinegar, wine,. There is only one phase of matter observed in a homogeneous mixture. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — many common liquids are homogeneous mixtures. — the components of a mixture retain their own properties and can be solid, liquid or gas. describe the solid, liquid and gas phases. Examples include steel, the gases in a scuba tank. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. — there is no partitional difference in the combination. Explain the difference between an element and. A homogeneous mixture appears uniform, regardless of where you sample it. No matter where you sample the.
From general.chemistrysteps.com
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps Homogeneous Mixture Solid Liquid And Gas Explain the difference between a pure substance and a mixture. Explain the difference between an element and. There is only one phase of matter observed in a homogeneous mixture. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. — there is no partitional difference. Homogeneous Mixture Solid Liquid And Gas.
From www.stfuandplay.com
Homogeneous Mixture Example Homogeneous Mixture Solid Liquid And Gas A homogeneous mixture is one that contains the same proportions of its. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. describe the solid, liquid and gas. Homogeneous Mixture Solid Liquid And Gas.
From www.differencebetween.com
Difference Between Homogeneous and Heterogeneous Equilibrium Compare Homogeneous Mixture Solid Liquid And Gas — there is no partitional difference in the combination. Examples include dishwashing liquid, shampoo, vinegar, wine,. Explain the difference between a pure substance and a mixture. — many common liquids are homogeneous mixtures. Examples include steel, the gases in a scuba tank. A homogeneous mixture is one that contains the same proportions of its. It is uniform in. Homogeneous Mixture Solid Liquid And Gas.
From lessondbunreverent.z21.web.core.windows.net
How To Calculate Liquid Density Homogeneous Mixture Solid Liquid And Gas — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. — many common liquids are homogeneous mixtures. It is uniform in composition throughout. A homogeneous mixture appears uniform, regardless of where you sample it. A homogeneous mixture is one that contains the same proportions of. Homogeneous Mixture Solid Liquid And Gas.
From primaryleap.co.uk
Chemistry Solutions And Mixtures Level 2 activity for kids Homogeneous Mixture Solid Liquid And Gas — a mixture is formed by combining two or more materials. — there is no partitional difference in the combination. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — many common liquids are homogeneous mixtures. There is only one phase of matter observed in a homogeneous mixture. A. Homogeneous Mixture Solid Liquid And Gas.
From themasterchemistry.com
15 Examples Of Homogeneous Mixtures Homogeneous Mixture Solid Liquid And Gas Explain the difference between a pure substance and a mixture. There is only one phase of matter observed in a homogeneous mixture. Examples include dishwashing liquid, shampoo, vinegar, wine,. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. Examples include steel, the gases in a. Homogeneous Mixture Solid Liquid And Gas.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID3016356 Homogeneous Mixture Solid Liquid And Gas Explain the difference between an element and. It is uniform in composition throughout. — a mixture is formed by combining two or more materials. describe the solid, liquid and gas phases. — there is no partitional difference in the combination. A homogeneous mixture is one that contains the same proportions of its. No matter where you sample. Homogeneous Mixture Solid Liquid And Gas.
From brainly.ph
Heterogeneous mixtures liquidgas examples Brainly.ph Homogeneous Mixture Solid Liquid And Gas — there is no partitional difference in the combination. A homogeneous mixture appears uniform, regardless of where you sample it. describe the solid, liquid and gas phases. Examples include dishwashing liquid, shampoo, vinegar, wine,. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample.. Homogeneous Mixture Solid Liquid And Gas.
From ar.inspiredpencil.com
Examples Of Homogeneous Mixtures Homogeneous Mixture Solid Liquid And Gas It is uniform in composition throughout. Explain the difference between a pure substance and a mixture. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — there is no partitional difference in the combination. — many common liquids are homogeneous mixtures. — a mixture is formed by combining two. Homogeneous Mixture Solid Liquid And Gas.
From www.youtube.com
SOLID LIQUID MIXTURE YouTube Homogeneous Mixture Solid Liquid And Gas — many common liquids are homogeneous mixtures. Explain the difference between a pure substance and a mixture. A homogeneous mixture appears uniform, regardless of where you sample it. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. No matter where you sample the. describe the solid, liquid and gas phases.. Homogeneous Mixture Solid Liquid And Gas.
From www.stfuandplay.com
Homogeneous Mixture Homogeneous Mixture Solid Liquid And Gas A homogeneous mixture is one that contains the same proportions of its. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. There is only one phase of matter observed in a homogeneous mixture. — a mixture is formed by combining two or more materials.. Homogeneous Mixture Solid Liquid And Gas.
From smartclass4kids.com
What is Mixture, Homogeneous Mixture, Heterogeneous Mixture with Examples Homogeneous Mixture Solid Liquid And Gas No matter where you sample the. describe the solid, liquid and gas phases. Explain the difference between a pure substance and a mixture. — a mixture is formed by combining two or more materials. There is only one phase of matter observed in a homogeneous mixture. — the components of a mixture retain their own properties and. Homogeneous Mixture Solid Liquid And Gas.
From www.chemicals.co.uk
What is a Mixture in Chemistry? The Chemistry Blog Homogeneous Mixture Solid Liquid And Gas No matter where you sample the. Examples include steel, the gases in a scuba tank. — there is no partitional difference in the combination. It is uniform in composition throughout. — a mixture is formed by combining two or more materials. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition.. Homogeneous Mixture Solid Liquid And Gas.
From examples.yourdictionary.com
Examples of Homogeneous Mixtures Solid, Liquid and Gas YourDictionary Homogeneous Mixture Solid Liquid And Gas There is only one phase of matter observed in a homogeneous mixture. Explain the difference between a pure substance and a mixture. — the components of a mixture retain their own properties and can be solid, liquid or gas. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. Examples include steel,. Homogeneous Mixture Solid Liquid And Gas.
From stock.adobe.com
illustration of chemistry, Pure Substances and Mixtures, element Homogeneous Mixture Solid Liquid And Gas — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — the components of a mixture retain their own properties and can be solid, liquid or gas. Examples include dishwashing liquid, shampoo, vinegar, wine,. It is uniform in composition throughout. Explain the difference between a pure substance and a mixture. A homogeneous. Homogeneous Mixture Solid Liquid And Gas.
From www.slideserve.com
PPT Mixtures of Matter PowerPoint Presentation, free download ID834762 Homogeneous Mixture Solid Liquid And Gas — there is no partitional difference in the combination. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. — a mixture is formed by combining two or more materials. A homogeneous mixture is one that contains the same proportions of its. No matter. Homogeneous Mixture Solid Liquid And Gas.
From studylibstearine.z21.web.core.windows.net
How To Separate Heterogeneous Mixtures Homogeneous Mixture Solid Liquid And Gas — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. Explain the difference between a pure substance and a mixture. Examples include dishwashing liquid, shampoo, vinegar, wine,. No matter where you sample the. — a homogeneous mixture is a solid, liquid, or gaseous mixture that. Homogeneous Mixture Solid Liquid And Gas.
From sciencenotes.org
10 Examples of Mixtures Homogeneous Mixture Solid Liquid And Gas A homogeneous mixture is one that contains the same proportions of its. — the components of a mixture retain their own properties and can be solid, liquid or gas. A homogeneous mixture appears uniform, regardless of where you sample it. describe the solid, liquid and gas phases. Examples include dishwashing liquid, shampoo, vinegar, wine,. — a homogeneous. Homogeneous Mixture Solid Liquid And Gas.
From www.dreamstime.com
Solution. Solid in liquid stock vector. Illustration of expansion Homogeneous Mixture Solid Liquid And Gas Examples include steel, the gases in a scuba tank. A homogeneous mixture appears uniform, regardless of where you sample it. It is uniform in composition throughout. — there is no partitional difference in the combination. A homogeneous mixture is one that contains the same proportions of its. — a mixture is formed by combining two or more materials.. Homogeneous Mixture Solid Liquid And Gas.
From chemistry.about.com
10 Heterogeneous and Homogeneous Mixtures Homogeneous Mixture Solid Liquid And Gas Explain the difference between an element and. It is uniform in composition throughout. — a mixture is formed by combining two or more materials. A homogeneous mixture appears uniform, regardless of where you sample it. Examples include dishwashing liquid, shampoo, vinegar, wine,. — many common liquids are homogeneous mixtures. — a homogeneous mixture is a solid, liquid,. Homogeneous Mixture Solid Liquid And Gas.
From primaryleap.co.uk
Chemistry Solutions And Mixtures Level 1 activity for kids Homogeneous Mixture Solid Liquid And Gas — the components of a mixture retain their own properties and can be solid, liquid or gas. There is only one phase of matter observed in a homogeneous mixture. It is uniform in composition throughout. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. Explain the difference between an element and.. Homogeneous Mixture Solid Liquid And Gas.
From bulleintime.com
Example Of A Gas Liquid Mixture Homogeneous Mixture Solid Liquid And Gas — the components of a mixture retain their own properties and can be solid, liquid or gas. No matter where you sample the. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. A homogeneous mixture is one that contains the same proportions of its. It is uniform in composition throughout. . Homogeneous Mixture Solid Liquid And Gas.
From themasterchemistry.com
Heterogeneous Mixture Example30 Fact Checked Homogeneous Mixture Solid Liquid And Gas — there is no partitional difference in the combination. Examples include dishwashing liquid, shampoo, vinegar, wine,. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. There is only one phase of matter observed in a homogeneous mixture. It is uniform in composition throughout. . Homogeneous Mixture Solid Liquid And Gas.
From www.dreamstime.com
Homogeneous Vs Heterogeneous Mixture Physical Properties Outline Homogeneous Mixture Solid Liquid And Gas Examples include steel, the gases in a scuba tank. Explain the difference between an element and. — there is no partitional difference in the combination. Examples include dishwashing liquid, shampoo, vinegar, wine,. There is only one phase of matter observed in a homogeneous mixture. Explain the difference between a pure substance and a mixture. A homogeneous mixture appears uniform,. Homogeneous Mixture Solid Liquid And Gas.
From www.youtube.com
SCIENCE 6 The two types of Mixtures Homogeneous Mixture and Homogeneous Mixture Solid Liquid And Gas Explain the difference between a pure substance and a mixture. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — there is no partitional difference in the combination. Examples include steel, the gases in a scuba tank. Explain the difference between an element and. A homogeneous mixture appears uniform, regardless of. Homogeneous Mixture Solid Liquid And Gas.
From proper-cooking.info
Heterogeneous Mixture Vs Homogeneous Mixture Homogeneous Mixture Solid Liquid And Gas — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. describe the solid, liquid and gas phases. — a mixture is formed by combining two or more materials. Examples include dishwashing liquid, shampoo, vinegar, wine,. — many common liquids are homogeneous mixtures. Examples. Homogeneous Mixture Solid Liquid And Gas.
From mungfali.com
Element Compound And Mixture Chart Homogeneous Mixture Solid Liquid And Gas — the components of a mixture retain their own properties and can be solid, liquid or gas. — there is no partitional difference in the combination. There is only one phase of matter observed in a homogeneous mixture. — a mixture is formed by combining two or more materials. A homogeneous mixture appears uniform, regardless of where. Homogeneous Mixture Solid Liquid And Gas.
From www.youtube.com
Science Lesson 4 Mixing Solids into Liquids YouTube Homogeneous Mixture Solid Liquid And Gas — there is no partitional difference in the combination. Examples include steel, the gases in a scuba tank. A homogeneous mixture appears uniform, regardless of where you sample it. — many common liquids are homogeneous mixtures. Examples include dishwashing liquid, shampoo, vinegar, wine,. Explain the difference between a pure substance and a mixture. — a homogeneous mixture. Homogeneous Mixture Solid Liquid And Gas.
From circuitdbhomemade.z13.web.core.windows.net
Homogeneous And Homogeneous Mixtures Homogeneous Mixture Solid Liquid And Gas There is only one phase of matter observed in a homogeneous mixture. — a mixture is formed by combining two or more materials. A homogeneous mixture is one that contains the same proportions of its. describe the solid, liquid and gas phases. Explain the difference between a pure substance and a mixture. Explain the difference between an element. Homogeneous Mixture Solid Liquid And Gas.
From giokwmczu.blob.core.windows.net
Solid Liquid And Gas Mixture Examples at Blake Cates blog Homogeneous Mixture Solid Liquid And Gas There is only one phase of matter observed in a homogeneous mixture. — the components of a mixture retain their own properties and can be solid, liquid or gas. Examples include dishwashing liquid, shampoo, vinegar, wine,. A homogeneous mixture is one that contains the same proportions of its. No matter where you sample the. Examples include steel, the gases. Homogeneous Mixture Solid Liquid And Gas.
From www.teachoo.com
Differentiate b/w Homogeneous and Heterogeneous mixtures Teachoo Homogeneous Mixture Solid Liquid And Gas Explain the difference between an element and. It is uniform in composition throughout. — the components of a mixture retain their own properties and can be solid, liquid or gas. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. Examples include dishwashing liquid, shampoo, vinegar, wine,. No matter where you sample. Homogeneous Mixture Solid Liquid And Gas.
From www.thoughtco.com
10 Heterogeneous and Homogeneous Mixtures Homogeneous Mixture Solid Liquid And Gas — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. describe the solid, liquid and gas phases. — the components of a mixture retain their own properties and can be solid, liquid or gas. — many common liquids are homogeneous mixtures. Examples include. Homogeneous Mixture Solid Liquid And Gas.
From www.thoughtco.com
Definition of Heterogeneous Mixture With Examples Homogeneous Mixture Solid Liquid And Gas — the components of a mixture retain their own properties and can be solid, liquid or gas. Explain the difference between an element and. — many common liquids are homogeneous mixtures. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. — a. Homogeneous Mixture Solid Liquid And Gas.
From sciencenotes.org
What Is a Homogeneous Mixture? Definition and Examples Homogeneous Mixture Solid Liquid And Gas Examples include dishwashing liquid, shampoo, vinegar, wine,. — a mixture is formed by combining two or more materials. — many common liquids are homogeneous mixtures. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of its components throughout a given sample. describe the solid, liquid and gas phases. Examples. Homogeneous Mixture Solid Liquid And Gas.
From dxotuvjtw.blob.core.windows.net
What Is Liquid Gas Mixture at Ruth Martinez blog Homogeneous Mixture Solid Liquid And Gas No matter where you sample the. — a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. — a mixture is formed by combining two or more materials. Examples include dishwashing liquid, shampoo, vinegar, wine,. — a homogeneous mixture is a gaseous, liquid or solid mixture that has the same proportions of. Homogeneous Mixture Solid Liquid And Gas.