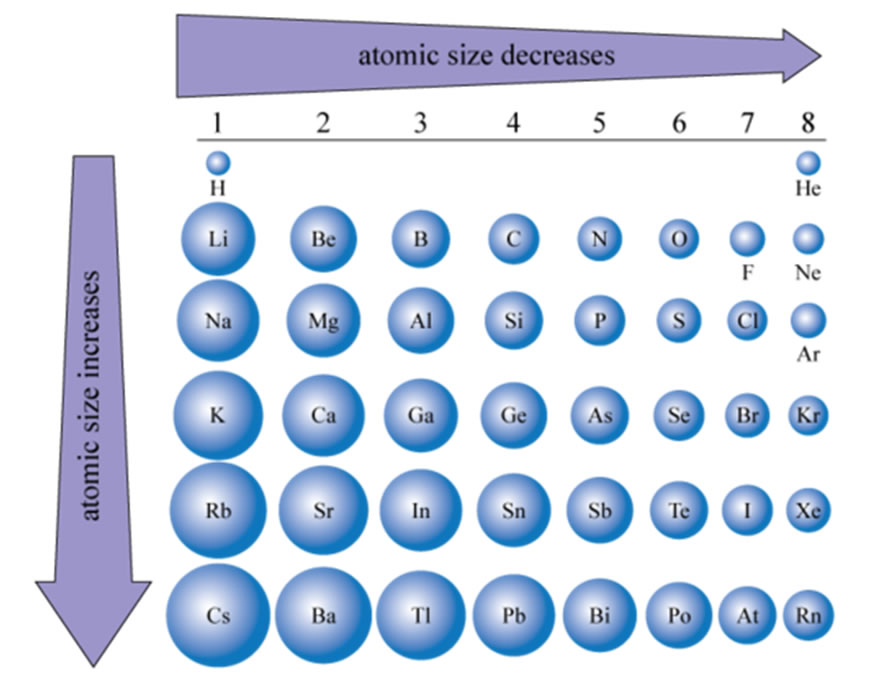
What Happens To The Number Of Shielding Electrons Across A Period . The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. The general trend is for ionisation energies to increase across a period. However there are breaks or variation in the trends in the. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. As z eff increases across a period, the ionization energy of the elements generally increases from left to right. Electrons in an s s orbital can shield p p. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on the. In a period due to the gradual change in electronic configuration across the period from member to member, there is a gradual change in. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____ it from the.
from www.chem.fsu.edu
Electrons in an s s orbital can shield p p. As z eff increases across a period, the ionization energy of the elements generally increases from left to right. The general trend is for ionisation energies to increase across a period. However there are breaks or variation in the trends in the. In a period due to the gradual change in electronic configuration across the period from member to member, there is a gradual change in. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on the. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____ it from the. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full.
Electron Configurations
What Happens To The Number Of Shielding Electrons Across A Period In a period due to the gradual change in electronic configuration across the period from member to member, there is a gradual change in. Electrons in an s s orbital can shield p p. However there are breaks or variation in the trends in the. The general trend is for ionisation energies to increase across a period. As z eff increases across a period, the ionization energy of the elements generally increases from left to right. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____ it from the. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on the. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. In a period due to the gradual change in electronic configuration across the period from member to member, there is a gradual change in. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in.
From klajgdeuu.blob.core.windows.net
Which Type Of Electrons Are Best At Shielding A 3P Electron at Tyra What Happens To The Number Of Shielding Electrons Across A Period The general trend is for ionisation energies to increase across a period. As z eff increases across a period, the ionization energy of the elements generally increases from left to right. However there are breaks or variation in the trends in the. The only way for ionization energy to increase across a period is if the only the number of. What Happens To The Number Of Shielding Electrons Across A Period.
From klajgdeuu.blob.core.windows.net
Which Type Of Electrons Are Best At Shielding A 3P Electron at Tyra What Happens To The Number Of Shielding Electrons Across A Period The general trend is for ionisation energies to increase across a period. However there are breaks or variation in the trends in the. Electrons in an s s orbital can shield p p. As z eff increases across a period, the ionization energy of the elements generally increases from left to right. The only way for ionization energy to increase. What Happens To The Number Of Shielding Electrons Across A Period.
From www.numerade.com
SOLVED correct options from the dropdown menus regarding Select the What Happens To The Number Of Shielding Electrons Across A Period The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. As z eff increases across a period, the ionization energy of the elements generally increases from left to right. However there are breaks or variation in the trends in the. The nuclear charge (zeff) experienced by an electron. What Happens To The Number Of Shielding Electrons Across A Period.
From slideplayer.com
Coulomb’s Law PERIODIC TRENDS Nuclear Charge Electron Shielding ppt What Happens To The Number Of Shielding Electrons Across A Period Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. However there are breaks or variation in the trends in the. As z eff increases across a period, the ionization energy of the elements generally increases from left to. What Happens To The Number Of Shielding Electrons Across A Period.
From socratic.org
How does the number of valence electrons change from left to right What Happens To The Number Of Shielding Electrons Across A Period The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on the. Electrons in an s s orbital can shield p p. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons. What Happens To The Number Of Shielding Electrons Across A Period.
From www.nagwa.com
Question Video Identifying Why Atoms Get Smaller from Left to Right What Happens To The Number Of Shielding Electrons Across A Period The general trend is for ionisation energies to increase across a period. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. In a period due to the gradual change in electronic configuration across the period from member to. What Happens To The Number Of Shielding Electrons Across A Period.
From www.slideserve.com
PPT Periodic Variation in Physical Properties PowerPoint Presentation What Happens To The Number Of Shielding Electrons Across A Period Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on the. The nuclear charge. What Happens To The Number Of Shielding Electrons Across A Period.
From blog.prepscholar.com
Understanding Atomic Radius Trends The 2 Key Principles · PrepScholar What Happens To The Number Of Shielding Electrons Across A Period As z eff increases across a period, the ionization energy of the elements generally increases from left to right. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. However there are breaks or variation in the trends in the. In a period due to the gradual change. What Happens To The Number Of Shielding Electrons Across A Period.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Happens To The Number Of Shielding Electrons Across A Period Electrons in an s s orbital can shield p p. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and. What Happens To The Number Of Shielding Electrons Across A Period.
From chemistry.stackexchange.com
chemistry My book's claim about the shielding effect of s,p What Happens To The Number Of Shielding Electrons Across A Period Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. However there are breaks or variation in the trends in the. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number. What Happens To The Number Of Shielding Electrons Across A Period.
From www.shemmassianconsulting.com
Atoms and Periodic Trends for the MCAT Everything You Need to Know What Happens To The Number Of Shielding Electrons Across A Period Electrons in an s s orbital can shield p p. As z eff increases across a period, the ionization energy of the elements generally increases from left to right. The general trend is for ionisation energies to increase across a period. However there are breaks or variation in the trends in the. In a period due to the gradual change. What Happens To The Number Of Shielding Electrons Across A Period.
From ceuwfxbf.blob.core.windows.net
What Does A Shielding Electron at Pamela Mccarthy blog What Happens To The Number Of Shielding Electrons Across A Period The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on the. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. Electrons in an s s orbital can shield p p. Where \(z\). What Happens To The Number Of Shielding Electrons Across A Period.
From general.chemistrysteps.com
Ionization energy Chemistry Steps What Happens To The Number Of Shielding Electrons Across A Period Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. In a period due to the gradual. What Happens To The Number Of Shielding Electrons Across A Period.
From www.thoughtco.com
Easy To Use Chart of Periodic Table Trends What Happens To The Number Of Shielding Electrons Across A Period The general trend is for ionisation energies to increase across a period. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on the. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____. What Happens To The Number Of Shielding Electrons Across A Period.
From loeqsufar.blob.core.windows.net
Fe Element Electrons at Jack Nguyen blog What Happens To The Number Of Shielding Electrons Across A Period The general trend is for ionisation energies to increase across a period. Electrons in an s s orbital can shield p p. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____ it from the. The shielding effect is when the electrons in full inner shells repel electrons. What Happens To The Number Of Shielding Electrons Across A Period.
From byjus.com
how to calculate the shielding effect What Happens To The Number Of Shielding Electrons Across A Period Electrons in an s s orbital can shield p p. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and. What Happens To The Number Of Shielding Electrons Across A Period.
From www.chegg.com
Solved Part 1 What is the number of core (shielding) What Happens To The Number Of Shielding Electrons Across A Period Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. The nuclear charge (zeff) experienced by an. What Happens To The Number Of Shielding Electrons Across A Period.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians What Happens To The Number Of Shielding Electrons Across A Period Electrons in an s s orbital can shield p p. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. In a period due to the gradual change in electronic configuration across the period from member to member, there. What Happens To The Number Of Shielding Electrons Across A Period.
From www.studocu.com
Shielding of Electrons Chemistry V Lab Studocu What Happens To The Number Of Shielding Electrons Across A Period The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____ it from the. However there are breaks or variation in the trends in the. As z eff increases across a period, the ionization energy of the elements generally increases from left to right. The shielding effect is when. What Happens To The Number Of Shielding Electrons Across A Period.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Happens To The Number Of Shielding Electrons Across A Period However there are breaks or variation in the trends in the. As z eff increases across a period, the ionization energy of the elements generally increases from left to right. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____ it from the. The general trend is for. What Happens To The Number Of Shielding Electrons Across A Period.
From www.numerade.com
SOLVED "Which statement is true regarding Slater's rules and the What Happens To The Number Of Shielding Electrons Across A Period Electrons in an s s orbital can shield p p. In a period due to the gradual change in electronic configuration across the period from member to member, there is a gradual change in. The general trend is for ionisation energies to increase across a period. However there are breaks or variation in the trends in the. The shielding effect. What Happens To The Number Of Shielding Electrons Across A Period.
From mmaawarrhitam.blogspot.com
As Zeff Increases The Electron Experiencing That Zeff Will / Periodic What Happens To The Number Of Shielding Electrons Across A Period Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. However there are breaks or variation in the trends in the. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number. What Happens To The Number Of Shielding Electrons Across A Period.
From pressbooks.bccampus.ca
1.6 Periodic Variations in Element Properties Chemistry for What Happens To The Number Of Shielding Electrons Across A Period In a period due to the gradual change in electronic configuration across the period from member to member, there is a gradual change in. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on the. The general trend is for ionisation energies to increase across. What Happens To The Number Of Shielding Electrons Across A Period.
From www.numerade.com
SOLVED What is meant by the term "shielding of electrons" in an atom What Happens To The Number Of Shielding Electrons Across A Period Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____ it from the. The general trend is. What Happens To The Number Of Shielding Electrons Across A Period.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision What Happens To The Number Of Shielding Electrons Across A Period Electrons in an s s orbital can shield p p. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from. What Happens To The Number Of Shielding Electrons Across A Period.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download What Happens To The Number Of Shielding Electrons Across A Period The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____ it from the. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on the. The general trend is for ionisation energies to increase. What Happens To The Number Of Shielding Electrons Across A Period.
From www.youtube.com
How To Determine The Number of Paired and Unpaired Electrons YouTube What Happens To The Number Of Shielding Electrons Across A Period As z eff increases across a period, the ionization energy of the elements generally increases from left to right. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____ it from the. The general trend is for ionisation energies to increase across a period. The only way for. What Happens To The Number Of Shielding Electrons Across A Period.
From perso.numericable.fr
The electrons in the inner shells shield the electrons in the outer What Happens To The Number Of Shielding Electrons Across A Period In a period due to the gradual change in electronic configuration across the period from member to member, there is a gradual change in. However there are breaks or variation in the trends in the. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. Electrons in an. What Happens To The Number Of Shielding Electrons Across A Period.
From www.youtube.com
Electron shielding YouTube What Happens To The Number Of Shielding Electrons Across A Period Electrons in an s s orbital can shield p p. As z eff increases across a period, the ionization energy of the elements generally increases from left to right. However there are breaks or variation in the trends in the. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated. What Happens To The Number Of Shielding Electrons Across A Period.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table What Happens To The Number Of Shielding Electrons Across A Period Electrons in an s s orbital can shield p p. The general trend is for ionisation energies to increase across a period. The only way for ionization energy to increase across a period is if the only the number of protons and valence electrons have effect on the. The nuclear charge (zeff) experienced by an electron depends on the _______. What Happens To The Number Of Shielding Electrons Across A Period.
From www.chem.fsu.edu
Electron Configurations What Happens To The Number Of Shielding Electrons Across A Period Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. Electrons in an s s orbital can shield p p. In a period due to the gradual change in electronic configuration across the period from member to member, there. What Happens To The Number Of Shielding Electrons Across A Period.
From www.youtube.com
What does higher shielding effect mean Which element has the highest What Happens To The Number Of Shielding Electrons Across A Period Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. However there are breaks or variation in the trends in the. The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them. What Happens To The Number Of Shielding Electrons Across A Period.
From www.teachoo.com
Define atomic size. Give its unit of measurement. In modern periodic What Happens To The Number Of Shielding Electrons Across A Period Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in. The nuclear charge (zeff) experienced by an electron depends on the _______ from the _____ and the number of _____ that ____ it from the. The only way for. What Happens To The Number Of Shielding Electrons Across A Period.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Happens To The Number Of Shielding Electrons Across A Period As z eff increases across a period, the ionization energy of the elements generally increases from left to right. Electrons in an s s orbital can shield p p. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus and the electron in.. What Happens To The Number Of Shielding Electrons Across A Period.
From scienceinfo.com
Shielding effect What Happens To The Number Of Shielding Electrons Across A Period The shielding effect is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the full. However there are breaks or variation in the trends in the. Where \(z\) is the atomic number (number of protons in nucleus) and \(s\) is the shielding constant and is approximated by number of electrons between the nucleus. What Happens To The Number Of Shielding Electrons Across A Period.