Facts About Buffer Solutions . — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. this page describes simple acidic and alkaline buffer solutions and explains how they work. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. What is a buffer solution?
from slidetodoc.com
A buffer solution is one which. — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. this page describes simple acidic and alkaline buffer solutions and explains how they work. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. What is a buffer solution?
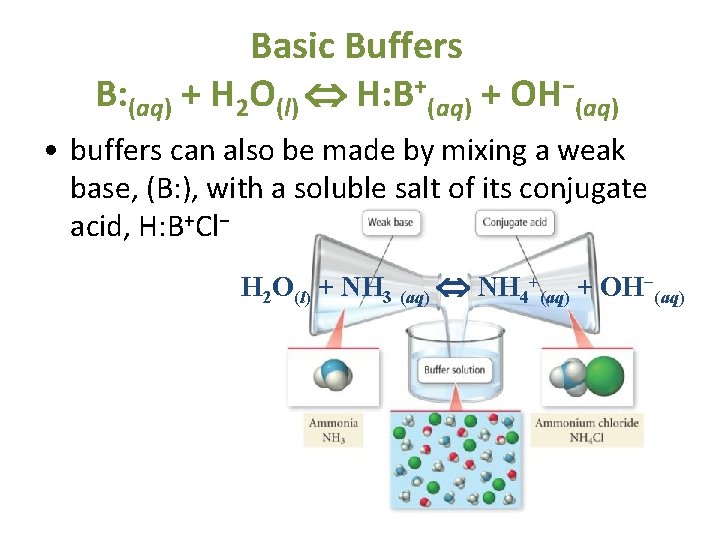
Introduction to Buffers These solutions contain relatively high
Facts About Buffer Solutions — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. this page describes simple acidic and alkaline buffer solutions and explains how they work. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. A buffer solution is one which. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. What is a buffer solution?
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses Facts About Buffer Solutions A buffer solution is one which. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. What is a buffer solution? . Facts About Buffer Solutions.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Facts About Buffer Solutions What is a buffer solution? — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. this page describes simple acidic and. Facts About Buffer Solutions.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Facts About Buffer Solutions What is a buffer solution? — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. A buffer solution is one which. . Facts About Buffer Solutions.
From www.toppr.com
Buffer Solutions Definition, Types, Preparation, Examples and Videos Facts About Buffer Solutions — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which. — a buffer solution is. Facts About Buffer Solutions.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Facts About Buffer Solutions — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. A buffer solution is one which. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. What. Facts About Buffer Solutions.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Facts About Buffer Solutions — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. this page describes simple acidic and alkaline buffer solutions and explains. Facts About Buffer Solutions.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Facts About Buffer Solutions — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — a buffer solution is a. Facts About Buffer Solutions.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Facts About Buffer Solutions A buffer solution is one which. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. . Facts About Buffer Solutions.
From www.slideserve.com
PPT Hydrolysis of Salts and pH of Buffer Solutions PowerPoint Facts About Buffer Solutions — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. A buffer solution is one which. — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. What. Facts About Buffer Solutions.
From design.udlvirtual.edu.pe
What Is A Buffer Solution Design Talk Facts About Buffer Solutions this page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. What is a buffer solution? — buffer, in chemistry,. Facts About Buffer Solutions.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID Facts About Buffer Solutions — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. What is a buffer solution? A buffer solution is one which. — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. . Facts About Buffer Solutions.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download Facts About Buffer Solutions — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. What is a buffer solution? — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — in other words, a buffer. Facts About Buffer Solutions.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Facts About Buffer Solutions — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. A buffer solution is one which. — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. What. Facts About Buffer Solutions.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Facts About Buffer Solutions — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. this page describes simple acidic and alkaline buffer solutions and explains. Facts About Buffer Solutions.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Facts About Buffer Solutions — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. What is a buffer solution? A buffer. Facts About Buffer Solutions.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID1031156 Facts About Buffer Solutions — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. A buffer solution is one which. . Facts About Buffer Solutions.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID4273107 Facts About Buffer Solutions — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. this page describes simple acidic and alkaline buffer solutions and explains how they work. — a buffer is a solution that maintains the stability of a system’s ph level. Facts About Buffer Solutions.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Facts About Buffer Solutions — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. — buffer, in chemistry, solution usually containing an acid and a. Facts About Buffer Solutions.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution Facts About Buffer Solutions this page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — a buffer is a solution that maintains the stability. Facts About Buffer Solutions.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Facts About Buffer Solutions A buffer solution is one which. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. — in other words, a. Facts About Buffer Solutions.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID Facts About Buffer Solutions — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. — a buffer is a solution. Facts About Buffer Solutions.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID691391 Facts About Buffer Solutions A buffer solution is one which. this page describes simple acidic and alkaline buffer solutions and explains how they work. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — buffer, in chemistry, solution usually containing an acid. Facts About Buffer Solutions.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Facts About Buffer Solutions this page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — a buffer solution is a carefully crafted mixture that. Facts About Buffer Solutions.
From peacecommission.kdsg.gov.ng
Buffer Solutions Definition, Types, Mechanisms, Uses Embibe Facts About Buffer Solutions A buffer solution is one which. — buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. this page describes simple acidic and alkaline buffer solutions and explains how they work. — a buffer is a solution that maintains the stability of a system’s ph. Facts About Buffer Solutions.
From www.pinterest.com.au
Buffer solutions Buffer solution, Chemistry lessons, Ap chemistry Facts About Buffer Solutions A buffer solution is one which. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. What is a buffer solution? . Facts About Buffer Solutions.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Facts About Buffer Solutions What is a buffer solution? — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. A buffer solution is one which. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. . Facts About Buffer Solutions.
From www.slideserve.com
PPT BUFFER SOLUTIONS A guide for A level students PowerPoint Facts About Buffer Solutions — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. A buffer solution is one which. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. . Facts About Buffer Solutions.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 Facts About Buffer Solutions — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. What is a buffer solution? —. Facts About Buffer Solutions.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Facts About Buffer Solutions — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a buffer solution? — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. —. Facts About Buffer Solutions.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Facts About Buffer Solutions What is a buffer solution? A buffer solution is one which. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. Facts About Buffer Solutions.
From www.youtube.com
What is Buffer Solution Types of Buffer Solution Acidic Buffer Facts About Buffer Solutions A buffer solution is one which. — a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. . Facts About Buffer Solutions.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Facts About Buffer Solutions this page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — a buffer is a solution that maintains the stability. Facts About Buffer Solutions.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high Facts About Buffer Solutions A buffer solution is one which. What is a buffer solution? — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. this page describes simple acidic and alkaline buffer solutions and explains how they work. — a buffer solution. Facts About Buffer Solutions.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Facts About Buffer Solutions this page describes simple acidic and alkaline buffer solutions and explains how they work. — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. A buffer solution is one which. — buffer, in chemistry, solution usually containing an acid. Facts About Buffer Solutions.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Facts About Buffer Solutions — in other words, a buffer solution (also known as a ph buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its. — a buffer solution is a carefully crafted mixture that helps maintain a stable ph level, even when an acid or base is. — buffer, in chemistry, solution usually. Facts About Buffer Solutions.