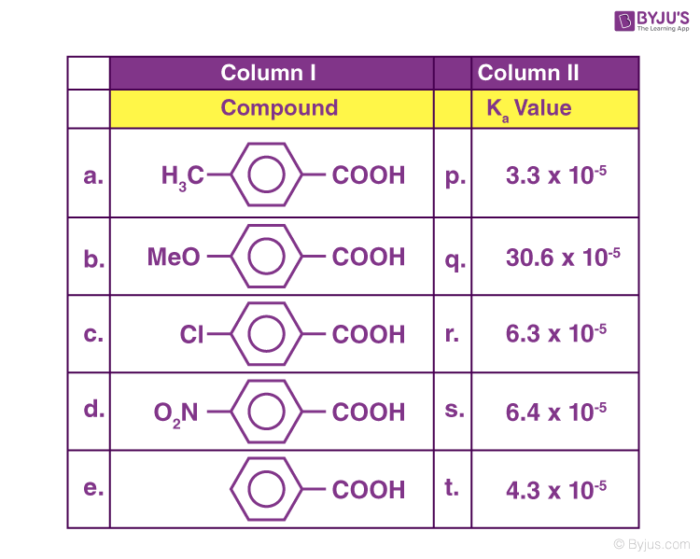
Induction Effects . the inductive effect is a consequence of the presence of electronegative atoms in a molecule. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive.
from byjus.com
the inductive effect is a consequence of the presence of electronegative atoms in a molecule. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive.
Inductive Effect Types of Inductive Effect, Applications, Stability
Induction Effects in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is a consequence of the presence of electronegative atoms in a molecule.
From byjus.com
Inductive Effect Types of Inductive Effect, Applications, Stability Induction Effects the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. the inductive effect is a consequence of the presence of electronegative atoms in a molecule. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in. Induction Effects.
From www.researchgate.net
induction effect at four typical situations. Download Induction Effects the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. the inductive effect is a consequence of the presence of electronegative atoms in a molecule. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive. Induction Effects.
From www.slideserve.com
PPT Conclusion Chapter 20 & Chapter 21 induction Induction Effects This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. with. Induction Effects.
From www.slideserve.com
PPT Induction PowerPoint Presentation, free download Induction Effects in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to. Induction Effects.
From leah4sci.com
Inductive Effect on Acid Base Strength in Organic Chemistry Induction Effects the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is a. Induction Effects.
From www.youtube.com
What is Inductive Effect ? YouTube Induction Effects the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. the inductive effect is a consequence of the presence of electronegative atoms in a molecule. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. with each substitution of cl for h, the acid becomes. Induction Effects.
From www.youtube.com
04.06 Stability Factors Inductive Effects YouTube Induction Effects with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. the inductive effect is a consequence of. Induction Effects.
From www.canarymedia.com
How do induction stoves actually work? Canary Media Induction Effects the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is crucial. Induction Effects.
From studylib.net
Inductive Effect Induction Effects with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. the inductive effect is crucial in determining. Induction Effects.
From chemistnotes.com
Inductive Effect Definitions/ Examples Chemistry Notes Induction Effects in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due. Induction Effects.
From www.youtube.com
Inductive Effect Basic Principle and Techniques in Organic Chemistry Induction Effects This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with. Induction Effects.
From www.slideserve.com
PPT CHAPTER 15 PowerPoint Presentation, free download ID2968807 Induction Effects This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the. Induction Effects.
From www.slideserve.com
PPT Induction PowerPoint Presentation ID228996 Induction Effects in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to. Induction Effects.
From www.youtube.com
inductive effect and its applications, organic chemistry YouTube Induction Effects with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is crucial in determining the behaviour of molecules. Induction Effects.
From slideplayer.com
Induction Fall /12/2018 Induction Fall ppt download Induction Effects in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. This effect can arise in sigma. Induction Effects.
From byjus.com
Inductive Effect Types of Inductive Effect, Applications, Stability Induction Effects This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. with each substitution of cl for h, the acid becomes stronger due to the shift of electrons,. Induction Effects.
From pdfprof.com
explain inductive effect Induction Effects This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. the inductive effect is a consequence of the presence of electronegative atoms in a molecule. with each substitution of cl for h, the acid becomes. Induction Effects.
From www.youtube.com
Comparing Acidities Inductive Effects YouTube Induction Effects This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. with. Induction Effects.
From thechemistrynotes.com
Inductive Effect Types, Uses, Stability Induction Effects the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the inductive effect is crucial in determining the behaviour of molecules in various. Induction Effects.
From www.teachoo.com
Induction Definition, Principle Class 10 Physics Induction Effects the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in. Induction Effects.
From www.youtube.com
Physics Understanding induction (EMI) and Induction Effects in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the. Induction Effects.
From www.youtube.com
Inductive Effect Reaction Mechanisms YouTube Induction Effects the inductive effect is a consequence of the presence of electronegative atoms in a molecule. with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. in this article. Induction Effects.
From discover.hubpages.com
Complete Chemistry of Inductive Effect Observed in Organic Molecule Induction Effects in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. the inductive effect is a. Induction Effects.
From www.youtube.com
Inductive effectpositive and negative inductive effectAcidity and Induction Effects in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. with each substitution of cl for h, the acid becomes stronger due to the shift of electrons,. Induction Effects.
From www.youtube.com
27.02 Resonance and Inductive Effects YouTube Induction Effects This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. the inductive effect is a consequence of the presence of electronegative atoms in. Induction Effects.
From www.youtube.com
Mutual Induction Animated explanation Induction Induction Effects the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. the inductive effect is a consequence of the presence of electronegative atoms in a molecule. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the inductive effect refers to the phenomenon wherein a permanent. Induction Effects.
From eduinput.com
InductionDefinition, Types, And Application Induction Effects the inductive effect is a consequence of the presence of electronegative atoms in a molecule. with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal. Induction Effects.
From www.youtube.com
Induction vs Resonance (Rules of Organic Chemistry 4) YouTube Induction Effects the inductive effect is a consequence of the presence of electronegative atoms in a molecule. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in. Induction Effects.
From www.pinterest.com
Faraday's law of induction vector illustration Electromotive force Induction Effects with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is a consequence of the presence of electronegative. Induction Effects.
From www.artofit.org
Induction heating theory Artofit Induction Effects the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the. Induction Effects.
From www.superprof.co.uk
Induction in Power Generation Superprof Induction Effects the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the. Induction Effects.
From www.slideserve.com
PPT Faraday’s Law and Lenz’s Law PowerPoint Presentation, free Induction Effects in this article we cover what is an inductive effect, how it occurs, two types of inductive effect with their examples, and finally how. the inductive effect is a consequence of the presence of electronegative atoms in a molecule. with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which. Induction Effects.
From www.slideserve.com
PPT INDUCTION PowerPoint Presentation, free download Induction Effects This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. the inductive effect is crucial in determining the behaviour of molecules in various. Induction Effects.
From www.slideserve.com
PPT Faraday’s Law and Lenz’s Law PowerPoint Presentation, free Induction Effects with each substitution of cl for h, the acid becomes stronger due to the shift of electrons, which is known as inductive. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. the inductive effect is crucial in determining the behaviour of molecules in various chemical reactions. in this article. Induction Effects.
From www.youtube.com
Inductive Effect Organic Chemistry YouTube Induction Effects the inductive effect refers to the phenomenon wherein a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds. in this article we cover what is an inductive effect, how it occurs,. Induction Effects.