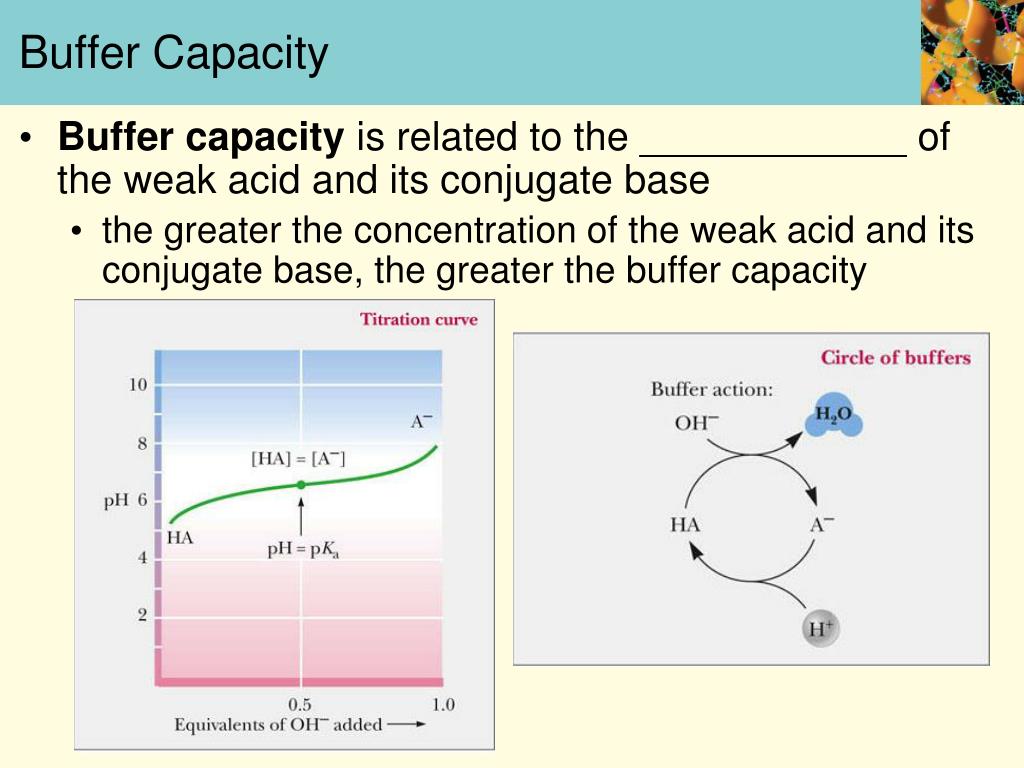
Buffer Capacity Vs Buffer Range . the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. buffers are characterized by two parameters, buffer range and buffer capacity. calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffer range is the interval of ph values over which a buffer can reliably neutralize. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. — buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to.
from www.slideserve.com
Buffer range is the interval of ph values over which a buffer can reliably neutralize. buffers are characterized by two parameters, buffer range and buffer capacity. although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. calculate the amounts of acid and base needed to prepare a buffer of a given ph. buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. — buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly.
PPT Chapter Two Water The Solvent for Biochemical Reactions
Buffer Capacity Vs Buffer Range Buffer range is the interval of ph values over which a buffer can reliably neutralize. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. buffers are characterized by two parameters, buffer range and buffer capacity. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. calculate the amounts of acid and base needed to prepare a buffer of a given ph. — buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to. buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. Buffer range is the interval of ph values over which a buffer can reliably neutralize.
From www.differencebetween.com
Difference Between Buffer Action and Buffer Capacity Compare the Buffer Capacity Vs Buffer Range buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffer range is the interval of ph values over which a buffer can reliably neutralize. — buffer capacity refers. Buffer Capacity Vs Buffer Range.
From studylib.net
Buffer Capacity Buffer Capacity Vs Buffer Range buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to. calculate the amounts of acid and base needed to prepare a buffer. Buffer Capacity Vs Buffer Range.
From study.com
Identifying Buffer Capacity Chemistry Buffer Capacity Vs Buffer Range calculate the amounts of acid and base needed to prepare a buffer of a given ph. — buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. buffers are characterized by two parameters, buffer range and buffer capacity. although the useful ph range. Buffer Capacity Vs Buffer Range.
From www.scribd.com
Sigma Buffer Chart Sigma Aldrich Tris Buffer Capacity Vs Buffer Range buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. Buffer range is the interval of ph values over which a buffer can reliably neutralize. buffers are characterized by two parameters, buffer range and buffer capacity. although the useful ph range of a buffer depends. Buffer Capacity Vs Buffer Range.
From www.difference.wiki
Buffer Action vs. Buffer Capacity What’s the Difference? Buffer Capacity Vs Buffer Range buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph. Buffer Capacity Vs Buffer Range.
From www.researchgate.net
Buffer capacity model specifications for a simulation interval between Buffer Capacity Vs Buffer Range Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Chapter 14 Equilibria in AcidBase Solutions PowerPoint Buffer Capacity Vs Buffer Range buffers are characterized by two parameters, buffer range and buffer capacity. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. calculate the amounts of acid and base needed. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Chapter 19 PowerPoint Presentation, free download ID4347161 Buffer Capacity Vs Buffer Range buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. calculate the amounts of acid and base needed to prepare a buffer of a given ph. — buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Chapter Two Water The Solvent for Biochemical Reactions Buffer Capacity Vs Buffer Range — buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. calculate the amounts of acid and base needed to prepare a buffer of a given ph. although the useful ph range of a buffer depends strongly on the chemical properties of the weak. Buffer Capacity Vs Buffer Range.
From www.engineeringtoolbox.com
Buffer solutions Buffer Capacity Vs Buffer Range although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Buffer Capacity Vs Buffer Range Buffer range is the interval of ph values over which a buffer can reliably neutralize. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. buffers are characterized by two parameters, buffer range and buffer capacity. although the useful ph range. Buffer Capacity Vs Buffer Range.
From www.youtube.com
Buffer range and capacity YouTube Buffer Capacity Vs Buffer Range Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak. Buffer Capacity Vs Buffer Range.
From courses.lumenlearning.com
Buffers Chemistry for Majors Buffer Capacity Vs Buffer Range calculate the amounts of acid and base needed to prepare a buffer of a given ph. — buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. buffer capacity is the amount of a strong acid or base a buffer can neutralize before a. Buffer Capacity Vs Buffer Range.
From thenoveldifference.com
Buffer Action and Buffer Capacity The best 4 difference Buffer Capacity Vs Buffer Range — buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. calculate the amounts of acid and base. Buffer Capacity Vs Buffer Range.
From pharmacyscope.com
How do you calculate buffer capacity? Pharmacy Scope Buffer Capacity Vs Buffer Range calculate the amounts of acid and base needed to prepare a buffer of a given ph. buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Buffer Capacity Vs Buffer Range the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be. Buffer Capacity Vs Buffer Range.
From 2012books.lardbucket.org
Buffers Buffer Capacity Vs Buffer Range the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. buffers are characterized by two parameters, buffer range and buffer capacity. although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak. Buffer Capacity Vs Buffer Range.
From dxoeuhryk.blob.core.windows.net
New Buffer Vs Buffer From at Kathryn Mitchell blog Buffer Capacity Vs Buffer Range calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffer range is the interval of ph values over which a buffer can reliably neutralize. although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to. — buffer capacity. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID6578454 Buffer Capacity Vs Buffer Range buffers are characterized by two parameters, buffer range and buffer capacity. calculate the amounts of acid and base needed to prepare a buffer of a given ph. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. — buffer capacity. Buffer Capacity Vs Buffer Range.
From www.youtube.com
Chapter 16. Buffers Buffer Range and Buffer Capacity YouTube Buffer Capacity Vs Buffer Range Buffer range is the interval of ph values over which a buffer can reliably neutralize. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. although the useful ph range of a buffer depends strongly on the chemical properties of the weak. Buffer Capacity Vs Buffer Range.
From www.studypool.com
SOLUTION 4 buffering capacity Studypool Buffer Capacity Vs Buffer Range Buffer range is the interval of ph values over which a buffer can reliably neutralize. buffers are characterized by two parameters, buffer range and buffer capacity. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be. Buffer Capacity Vs Buffer Range.
From www.youtube.com
Buffer Range and Buffer Capacity.mp4 YouTube Buffer Capacity Vs Buffer Range calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffer range is the interval of ph values over which a buffer can reliably neutralize. although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to. the buffer capacity. Buffer Capacity Vs Buffer Range.
From relationshipbetween.com
Difference Between Buffer Action And Buffer Capacity Relationship Between Buffer Capacity Vs Buffer Range calculate the amounts of acid and base needed to prepare a buffer of a given ph. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. buffer capacity is the amount of a strong acid or base a buffer can neutralize. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Buffer Capacity Vs Buffer Range Buffer range is the interval of ph values over which a buffer can reliably neutralize. buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. buffers are characterized by two parameters, buffer range and buffer capacity. Buffers are characterized by the ph range over which they. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Buffer Capacity Vs Buffer Range Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. — buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid. Buffer Capacity Vs Buffer Range.
From chem-for-fun.blogspot.com
CHEMISTRY IS NOT HORRIBLE Buffers and Buffering Capacity Buffer Capacity Vs Buffer Range buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to. Buffer range is the interval of ph values over which a buffer can. Buffer Capacity Vs Buffer Range.
From www.youtube.com
Buffer capacity Acids and bases AP Chemistry Khan Academy YouTube Buffer Capacity Vs Buffer Range calculate the amounts of acid and base needed to prepare a buffer of a given ph. buffers are characterized by two parameters, buffer range and buffer capacity. Buffer range is the interval of ph values over which a buffer can reliably neutralize. the buffer capacity is the amount of acid or base that can be added to. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 Buffer Capacity Vs Buffer Range buffers are characterized by two parameters, buffer range and buffer capacity. Buffer range is the interval of ph values over which a buffer can reliably neutralize. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. — buffer capacity refers to. Buffer Capacity Vs Buffer Range.
From exoxnpydf.blob.core.windows.net
Biological Buffer Chart at Megan Hankins blog Buffer Capacity Vs Buffer Range — buffer capacity refers to the ability of a buffer solution to resist changes in its ph when an acid or base is added. buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. the buffer capacity is the amount of acid or base that. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Buffer Capacity Vs Buffer Range Buffer range is the interval of ph values over which a buffer can reliably neutralize. buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer. Buffer Capacity Vs Buffer Range.
From slideplayer.com
principles and modern applications ppt download Buffer Capacity Vs Buffer Range Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of strong acid or base that can be absorbed before the ph changes significantly. the buffer capacity is the amount of acid or base that can be added to a given volume of a. Buffer Capacity Vs Buffer Range.
From www.researchgate.net
Buffering capacity β of (a) water, (b) ammonia or ammonium, and (c Buffer Capacity Vs Buffer Range calculate the amounts of acid and base needed to prepare a buffer of a given ph. buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. buffers are characterized by two parameters, buffer range and buffer capacity. the buffer capacity is the amount of. Buffer Capacity Vs Buffer Range.
From www.researchgate.net
6. Schematic diagram of the buffer capacity of an aqueous system Buffer Capacity Vs Buffer Range calculate the amounts of acid and base needed to prepare a buffer of a given ph. although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to. Buffer range is the interval of ph values over which a buffer can reliably neutralize. — buffer capacity. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Chapter Two Water The Solvent for Biochemical Reactions Buffer Capacity Vs Buffer Range buffers are characterized by two parameters, buffer range and buffer capacity. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. Buffer Capacity Vs Buffer Range.
From www.slideserve.com
PPT Buffer Capacity Lab PowerPoint Presentation, free download ID Buffer Capacity Vs Buffer Range buffer capacity is the amount of a strong acid or base a buffer can neutralize before a significant change in its ph. calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffer range is the interval of ph values over which a buffer can reliably neutralize. buffers are characterized by. Buffer Capacity Vs Buffer Range.