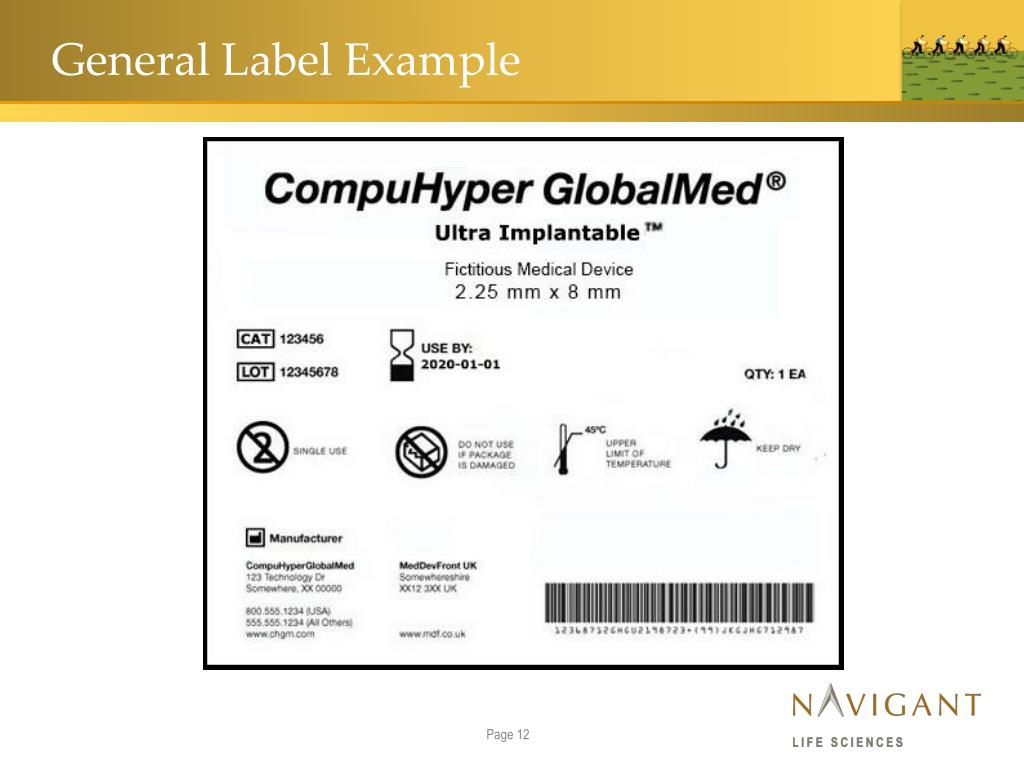
Fda Off Label Use Medical Device . The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. (1) register their establishments and list the medical devices they market with fda; The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. (2) manufacture their devices in accordance with. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and potentially save. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications.
from www.slideserve.com
(1) register their establishments and list the medical devices they market with fda; Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and potentially save. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. (2) manufacture their devices in accordance with. The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider.
PPT Medical Device Labeling PowerPoint Presentation, free download
Fda Off Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and potentially save. (2) manufacture their devices in accordance with. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. (1) register their establishments and list the medical devices they market with fda;
From www.audible.com
FDA's Role in the Off Label Use of Medical Devices Explained by Dr Fda Off Label Use Medical Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. (1) register their establishments and list the medical devices they market with fda; A pair of guidance documents outlines how medtech and pharmaceutical companies. Fda Off Label Use Medical Device.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Fda Off Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. (1) register their establishments and list the medical devices they market with fda; The preamble asserts that under ’s approach. Fda Off Label Use Medical Device.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Fda Off Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. (1) register their establishments and list the medical devices they market with fda; The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. On tuesday, october 24, fda announced the. Fda Off Label Use Medical Device.
From www.pm360online.com
What Does the FDA’s New Offlabel Guidance Mean for Pharma? PM360 Fda Off Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. (2) manufacture their devices. Fda Off Label Use Medical Device.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Fda Off Label Use Medical Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. A pair of guidance. Fda Off Label Use Medical Device.
From fra.animalia-life.club
Iso 15223 1 Symboles Fda Off Label Use Medical Device (2) manufacture their devices in accordance with. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and potentially save. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. The new rule and its accompanying fda preamble clarify that (1). Fda Off Label Use Medical Device.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Off Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. (1) register their establishments and list the medical devices they market with fda; (2) manufacture their devices in accordance with. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider.. Fda Off Label Use Medical Device.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Fda Off Label Use Medical Device The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and potentially save. On tuesday, october 24, fda announced the availability of a revised draft guidance document. Fda Off Label Use Medical Device.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Fda Off Label Use Medical Device (2) manufacture their devices in accordance with. The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain. Fda Off Label Use Medical Device.
From bioregservices.com
About Product Intended Use, Label and OffLabel Promotion for Medical Fda Off Label Use Medical Device A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and potentially save. (1) register their establishments and list the medical devices they market with fda; (2) manufacture their devices in accordance with. The new rule and its accompanying fda preamble clarify that (1) evidence of a. Fda Off Label Use Medical Device.
From www.vrogue.co
Fda Launches New page To Promote Use Of Symbols In vrogue.co Fda Off Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. (2) manufacture their devices. Fda Off Label Use Medical Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Off Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. (1) register their establishments and list the medical devices they market with fda; (2) manufacture their devices in accordance with.. Fda Off Label Use Medical Device.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Fda Off Label Use Medical Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. (2) manufacture their devices in accordance with. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and potentially save. Good medical practice and the best interests of the patient require. Fda Off Label Use Medical Device.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Off Label Use Medical Device (2) manufacture their devices in accordance with. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. On tuesday, october 24, fda announced the availability of a revised draft guidance. Fda Off Label Use Medical Device.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Off Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and potentially save. On tuesday, october 24, fda announced the availability of a revised draft guidance document. Fda Off Label Use Medical Device.
From www.druganddevicelawblog.com
Examining The Amarin/FDA OffLabel Promotion Settlement Drug & Device Law Fda Off Label Use Medical Device The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices.. Fda Off Label Use Medical Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Off Label Use Medical Device (1) register their establishments and list the medical devices they market with fda; The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. (2) manufacture their devices in accordance with. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider.. Fda Off Label Use Medical Device.
From mungfali.com
FDA Medical Device Label Symbols Fda Off Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. (2) manufacture their devices in accordance with. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. On tuesday, october 24, fda announced the availability of a revised draft guidance. Fda Off Label Use Medical Device.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Off Label Use Medical Device (2) manufacture their devices in accordance with. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. On tuesday, october 24, fda announced the availability of a revised draft guidance. Fda Off Label Use Medical Device.
From emmainternational.com
Discovering FDALabel Your GoTo Labelling Tool Fda Off Label Use Medical Device The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. (1) register their establishments and list the medical devices they market with fda; (2) manufacture their devices in accordance with. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. A pair of guidance. Fda Off Label Use Medical Device.
From www.pharmaceutical-technology.com
FDA cracks down on offlabel drug use messaging Pharmaceutical Technology Fda Off Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and potentially save. Good medical practice and the best interests of the patient require that physicians use. Fda Off Label Use Medical Device.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Off Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. (1) register their establishments and list the medical devices they market with fda; On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. The preamble asserts that under ’s approach to intended use, these. Fda Off Label Use Medical Device.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Fda Off Label Use Medical Device (1) register their establishments and list the medical devices they market with fda; (2) manufacture their devices in accordance with. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products. Fda Off Label Use Medical Device.
From mungfali.com
Medical Device Labeling Symbols Fda Off Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. (2) manufacture their devices in accordance with. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals. Fda Off Label Use Medical Device.
From www.slideshare.net
FDA Unique Device Identification (UDI) Overview Fda Off Label Use Medical Device (1) register their establishments and list the medical devices they market with fda; The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. The new rule and its accompanying fda. Fda Off Label Use Medical Device.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Off Label Use Medical Device (2) manufacture their devices in accordance with. The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. (1) register their establishments and list the medical devices they market with fda;. Fda Off Label Use Medical Device.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Off Label Use Medical Device A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and potentially save. (2) manufacture their devices in accordance with. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. The preamble asserts that under ’s approach. Fda Off Label Use Medical Device.
From www.northcarolinaproductliabilitylawyer.com
OffLabel Use Category Archives — North Carolina Product Liability Fda Off Label Use Medical Device (1) register their establishments and list the medical devices they market with fda; On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. A pair of guidance documents outlines how medtech and pharmaceutical companies. Fda Off Label Use Medical Device.
From www.youtube.com
Good OffLabel Use Practice of Medicines YouTube Fda Off Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and. Fda Off Label Use Medical Device.
From stellacenter.com
OffLabel Medical Treatments Proven Effective Stella Fda Off Label Use Medical Device (2) manufacture their devices in accordance with. (1) register their establishments and list the medical devices they market with fda; On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals how their products can affect outcomes and. Fda Off Label Use Medical Device.
From www.youtube.com
FDA's Role in the Off Label Use of Medical Devices Explained by Dr Fda Off Label Use Medical Device The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish a firm’s intent. The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. (1) register their establishments and list the medical devices they market with fda; On tuesday, october 24, fda announced the. Fda Off Label Use Medical Device.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Fda Off Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. (1) register their establishments and list the medical devices they market with fda; On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. A pair of guidance documents outlines how medtech and pharmaceutical companies. Fda Off Label Use Medical Device.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Off Label Use Medical Device The new rule and its accompanying fda preamble clarify that (1) evidence of a company’s knowledge that a healthcare provider. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. (1) register their establishments and list the medical devices they market with fda; The preamble asserts that under ’s approach. Fda Off Label Use Medical Device.
From studylib.net
OFFLABEL USE OF MEDICAL PRODUCTS WARRANTY AND Fda Off Label Use Medical Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. (2) manufacture their devices in accordance with. The preamble asserts that under ’s approach to intended use, these types of firm communications “,” establish. Fda Off Label Use Medical Device.
From www.medicaldesignandoutsourcing.com
FDA to clarify role of offlabel uses in medical device approvals Fda Off Label Use Medical Device Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. (2) manufacture their devices in accordance with. A pair of guidance documents outlines how medtech and pharmaceutical companies can explain to payors and hospitals. Fda Off Label Use Medical Device.