Heat Of Formation Of N2O5 . Nist chemistry webbook the national institute of. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. The heat of vaporisation of liquid methyl. Visit byju's to understand the properties, structure and its uses. Define molar enthalpy of formation of compounds. Calculate the molar enthalpy of formation from combustion data using hess's law. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data.
from www.chegg.com
Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Nist chemistry webbook the national institute of. The heat of vaporisation of liquid methyl. Define molar enthalpy of formation of compounds. Visit byju's to understand the properties, structure and its uses. Enthalpy of reaction at standard conditions data from nist standard reference database 69: A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Calculate the molar enthalpy of formation from combustion data using hess's law.
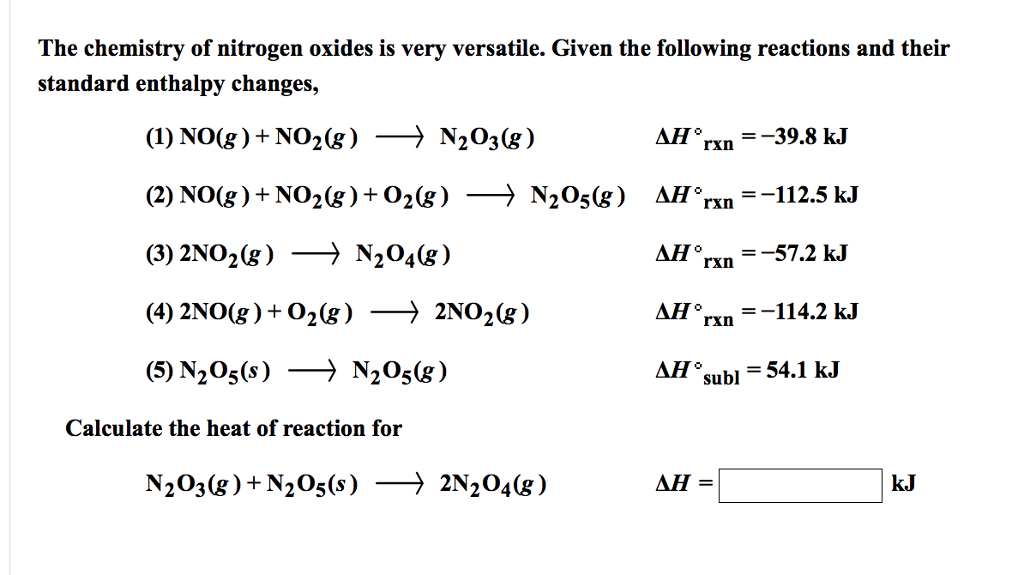
Solved The chemistry of nitrogen oxides is very versatile.
Heat Of Formation Of N2O5 Calculate the molar enthalpy of formation from combustion data using hess's law. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Nist chemistry webbook the national institute of. Calculate the molar enthalpy of formation from combustion data using hess's law. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. The heat of vaporisation of liquid methyl. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Visit byju's to understand the properties, structure and its uses. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Define molar enthalpy of formation of compounds.
From animalia-life.club
N2o5 Structure Heat Of Formation Of N2O5 Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Define molar enthalpy of formation of compounds. Nist chemistry webbook the national institute of. Visit byju's to understand. Heat Of Formation Of N2O5.
From www.chegg.com
Solved Calculate the standard enthalpy of formation of N2O5 Heat Of Formation Of N2O5 Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Define molar enthalpy of formation of compounds. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Calculate the molar enthalpy of formation. Heat Of Formation Of N2O5.
From www.researchgate.net
Chemical ionization mass spectrometer for N2O5 detection. CDC Heat Of Formation Of N2O5 Visit byju's to understand the properties, structure and its uses. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Define molar enthalpy of formation of compounds. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. A. Heat Of Formation Of N2O5.
From oneclass.com
OneClass Calculate the standard enthalpy of formation of N2O5 from the Heat Of Formation Of N2O5 Define molar enthalpy of formation of compounds. Calculate the molar enthalpy of formation from combustion data using hess's law. Visit byju's to understand the properties, structure and its uses. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Nist chemistry webbook the national institute of. The heat of vaporisation of liquid methyl.. Heat Of Formation Of N2O5.
From www.chegg.com
Solved Which of the following chemical equations corresponds Heat Of Formation Of N2O5 Calculate the molar enthalpy of formation from combustion data using hess's law. Nist chemistry webbook the national institute of. Enthalpy of reaction at standard conditions data from nist standard reference database 69: The heat of vaporisation of liquid methyl. Visit byju's to understand the properties, structure and its uses. Compute the heat of formation of liquid methyl alcohol in kilojoules. Heat Of Formation Of N2O5.
From www.chegg.com
10. The thermal of N2O5 occurs as 2 Heat Of Formation Of N2O5 Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. The heat of vaporisation of liquid methyl. Calculate the molar enthalpy of formation from combustion data using hess's law. Enthalpy of reaction at standard conditions data from nist standard reference database. Heat Of Formation Of N2O5.
From www.chegg.com
Solved (a) Given the thermochemical equations below, Heat Of Formation Of N2O5 The heat of vaporisation of liquid methyl. Visit byju's to understand the properties, structure and its uses. Calculate the molar enthalpy of formation from combustion data using hess's law. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Define. Heat Of Formation Of N2O5.
From www.chegg.com
Solved S The gasphase of N2O5 is a Heat Of Formation Of N2O5 Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Visit byju's to understand the properties, structure and its uses. A very simple way of obtaining nitrogen pentoxide. Heat Of Formation Of N2O5.
From www.numerade.com
SOLVED The of crystalline N2O5 N2O5(s) → 2NO2(g) + 1/2O2 Heat Of Formation Of N2O5 The heat of vaporisation of liquid methyl. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Define molar enthalpy of formation of. Heat Of Formation Of N2O5.
From www.toppr.com
If rate of formation of O2 is 16 g/hr, then rate of of Heat Of Formation Of N2O5 Calculate the molar enthalpy of formation from combustion data using hess's law. The heat of vaporisation of liquid methyl. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Define molar enthalpy of formation of compounds. Enthalpy of reaction at. Heat Of Formation Of N2O5.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Heat Of Formation Of N2O5 Visit byju's to understand the properties, structure and its uses. Nist chemistry webbook the national institute of. Define molar enthalpy of formation of compounds. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Calculate the molar enthalpy of formation. Heat Of Formation Of N2O5.
From byjus.com
22. The heat of formation (N2O5,g) in KJ/mol on the bases of the Heat Of Formation Of N2O5 Enthalpy of reaction at standard conditions data from nist standard reference database 69: Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Visit byju's to understand the properties, structure and its uses. Calculate the. Heat Of Formation Of N2O5.
From oneclass.com
OneClass Solve in details. 1. Calculate the enthalpy of the followving Heat Of Formation Of N2O5 Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Calculate the molar enthalpy of formation from combustion data using hess's law. Nist chemistry webbook the national institute of. The heat of vaporisation of liquid methyl. Compute the heat of. Heat Of Formation Of N2O5.
From www.youtube.com
Balancing the Equation N2O5 = N2O4 + O2 (and Type of Reaction) YouTube Heat Of Formation Of N2O5 Calculate the molar enthalpy of formation from combustion data using hess's law. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Define molar enthalpy of formation of compounds. Dinitrogen pentoxide is a powerful oxidising. Heat Of Formation Of N2O5.
From www.chegg.com
Solved b. Would enthalpy of formation for N2O5 (g) be the Heat Of Formation Of N2O5 Calculate the molar enthalpy of formation from combustion data using hess's law. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Nist chemistry webbook the national institute of. The heat of vaporisation of liquid methyl. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Dinitrogen pentoxide is a. Heat Of Formation Of N2O5.
From www.chegg.com
Solved 6. The overall reaction for the of N2O5 Heat Of Formation Of N2O5 Define molar enthalpy of formation of compounds. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Enthalpy of reaction at standard conditions data from nist standard reference database 69: A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Nist chemistry webbook the national institute of. The heat of. Heat Of Formation Of N2O5.
From studyschoolburman.z21.web.core.windows.net
How To Find The Heat Of Formation Heat Of Formation Of N2O5 The heat of vaporisation of liquid methyl. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Visit byju's to understand the properties, structure and its uses. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide. Heat Of Formation Of N2O5.
From byjus.com
For the reaction 2N2O5 > 4NO2 + O2 the rate of formation of O2 is 0 Heat Of Formation Of N2O5 Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Nist chemistry webbook the national institute of. The heat of vaporisation of liquid methyl. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Define molar enthalpy of formation of compounds. Visit byju's to understand the properties, structure and its. Heat Of Formation Of N2O5.
From mungfali.com
Standard Enthalpy Change Equation Heat Of Formation Of N2O5 A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. The heat of vaporisation of liquid methyl. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Nist chemistry webbook the national institute of. Define molar enthalpy of formation of compounds. Calculate the molar enthalpy of formation from combustion. Heat Of Formation Of N2O5.
From www.chegg.com
Solved Experiments show that if the chemical reaction Heat Of Formation Of N2O5 Nist chemistry webbook the national institute of. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Define molar enthalpy of formation of compounds. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the. Heat Of Formation Of N2O5.
From slideplayer.com
Reaction Rates and Equilibrium ppt download Heat Of Formation Of N2O5 Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Nist chemistry webbook the national institute of. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Dinitrogen pentoxide is a powerful. Heat Of Formation Of N2O5.
From www.researchgate.net
The enthalpy of formation of O 2 + and N 5 + , various anions and their Heat Of Formation Of N2O5 A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Calculate the molar enthalpy of formation from combustion data using hess's law. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Define molar enthalpy of formation of compounds. Nist chemistry webbook the national institute of. The heat of vaporisation. Heat Of Formation Of N2O5.
From www.chegg.com
Solved The of N2O5 can be described by the Heat Of Formation Of N2O5 The heat of vaporisation of liquid methyl. Define molar enthalpy of formation of compounds. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Enthalpy of reaction at standard conditions data from nist standard reference database 69: A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen. Heat Of Formation Of N2O5.
From www.chegg.com
Solved Exercise 4 The of N2O5 in the gas phase Heat Of Formation Of N2O5 Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Visit byju's to understand the properties, structure and its uses. Define molar enthalpy of formation of compounds. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Enthalpy of reaction at standard conditions data from. Heat Of Formation Of N2O5.
From byjus.com
66 Enthalpies of formation of co(g),CO2(g),N2O(g)and N2O4(g) are 110 Heat Of Formation Of N2O5 Calculate the molar enthalpy of formation from combustion data using hess's law. The heat of vaporisation of liquid methyl. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. A very simple way of obtaining nitrogen pentoxide involves the reaction of. Heat Of Formation Of N2O5.
From www.chegg.com
Solved 20. Consider the reaction 2N2O5(g)4NO2(g) + O2(g) at Heat Of Formation Of N2O5 Enthalpy of reaction at standard conditions data from nist standard reference database 69: Calculate the molar enthalpy of formation from combustion data using hess's law. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Nist chemistry webbook the national institute of. Visit byju's to understand the properties, structure and its uses. The. Heat Of Formation Of N2O5.
From byjus.com
For the reaction 2N2O5 > 4NO2 + O2 the rate of formation of O2 is 0 Heat Of Formation Of N2O5 Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data.. Heat Of Formation Of N2O5.
From www.numerade.com
SOLVED A proposed mechanism for the formation of N2O5 from NO2 and O3 Heat Of Formation Of N2O5 A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Calculate the molar enthalpy of formation from combustion data using hess's law. Nist chemistry webbook the national institute of. The heat of vaporisation of liquid methyl. Visit byju's to understand the properties, structure and its uses. Dinitrogen pentoxide is a powerful oxidising. Heat Of Formation Of N2O5.
From www.chegg.com
An exothermic liquid phase reaction employs N2O5 as Heat Of Formation Of N2O5 A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Visit byju's to understand the properties, structure and its uses. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Calculate the molar enthalpy of formation from combustion data using hess's law. Compute the heat of formation of liquid. Heat Of Formation Of N2O5.
From brainly.in
For the reaction; 2n2o5 4no2 + o2 rate and rate constant are 1.02 104 Heat Of Formation Of N2O5 Calculate the molar enthalpy of formation from combustion data using hess's law. The heat of vaporisation of liquid methyl. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Visit byju's to understand the properties, structure and its uses. Define. Heat Of Formation Of N2O5.
From www.numerade.com
SOLVED . Calculate the standard enthalpy of formation of dinitrogen Heat Of Formation Of N2O5 Visit byju's to understand the properties, structure and its uses. Nist chemistry webbook the national institute of. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. The heat of vaporisation of liquid methyl. Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. Calculate the molar enthalpy of formation from. Heat Of Formation Of N2O5.
From www.chegg.com
Solved The chemistry of nitrogen oxides is very versatile. Heat Of Formation Of N2O5 Define molar enthalpy of formation of compounds. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Nist chemistry webbook the national institute of. A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. The heat of vaporisation of liquid methyl. Compute the heat of formation of liquid methyl. Heat Of Formation Of N2O5.
From www.youtube.com
N2O5+H2O=HNO3 Balanced EquationNitrogen pentaoxide+Water=Nitric acid Heat Of Formation Of N2O5 Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. The heat of vaporisation of liquid methyl. Visit byju's to understand the properties, structure and its uses. Calculate. Heat Of Formation Of N2O5.
From www.toppr.com
Consider the reaction 4NO2(g) O2(g) → 2N2O5(g), Δ r H = 111 kJ If Heat Of Formation Of N2O5 Enthalpy of reaction at standard conditions data from nist standard reference database 69: A very simple way of obtaining nitrogen pentoxide involves the reaction of nitrogen dioxide or dinitrogen tetroxide with. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Define molar enthalpy of formation of compounds. Compute the heat of formation of liquid methyl alcohol in. Heat Of Formation Of N2O5.
From www.chegg.com
Solved Gaseous Nitrogen Dioxide NO2, Reacts With Oxygen T... Heat Of Formation Of N2O5 Nist chemistry webbook the national institute of. Dinitrogen pentoxide is a powerful oxidising agent with the chemical formula n2o5. Enthalpy of reaction at standard conditions data from nist standard reference database 69: Compute the heat of formation of liquid methyl alcohol in kilojoules per mole using the following data. A very simple way of obtaining nitrogen pentoxide involves the reaction. Heat Of Formation Of N2O5.