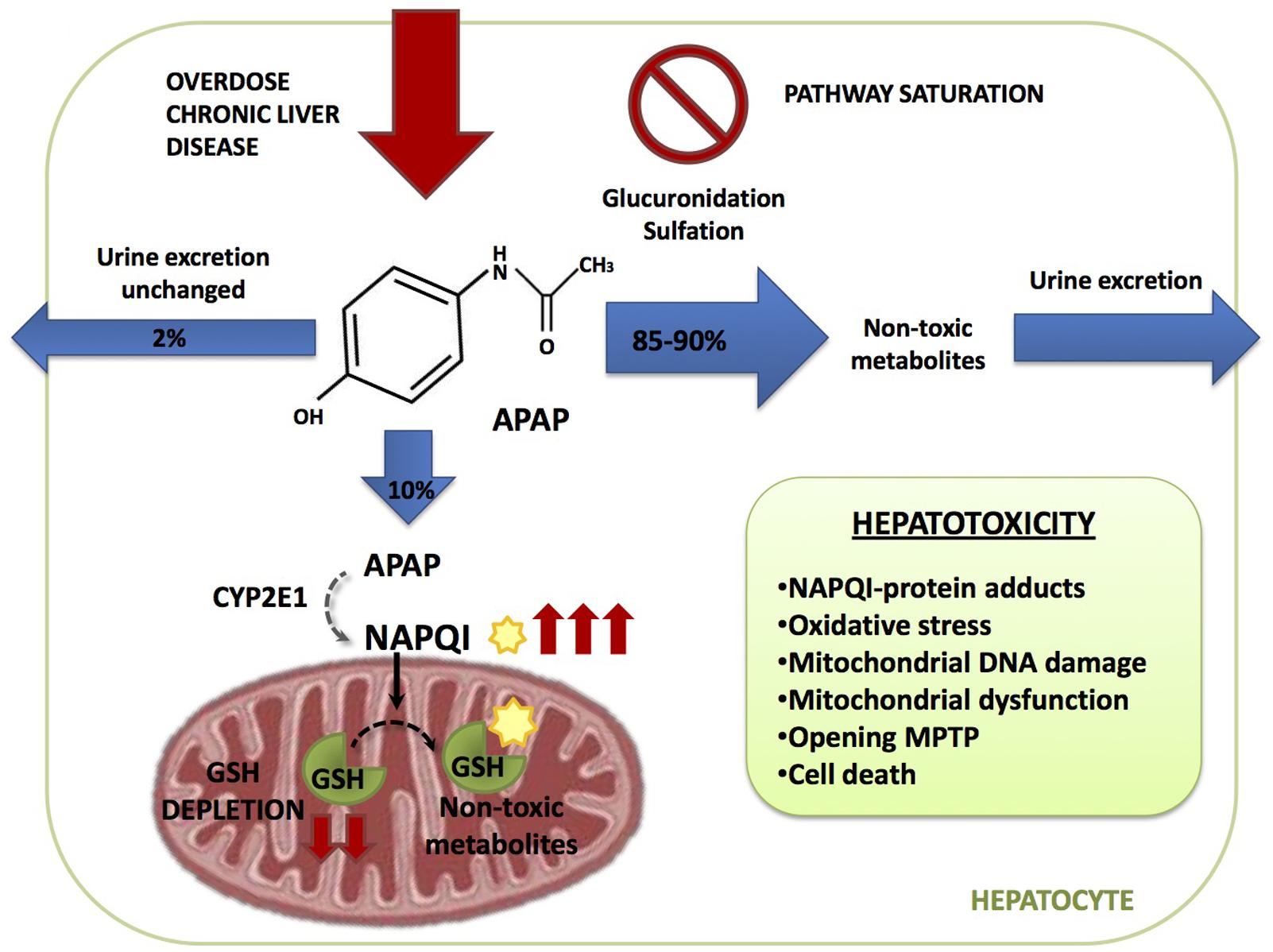
Paracetamol Hepatotoxicity In Pediatrics . “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. A single dose of 150. In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. Identify hepatotoxicity concerns and serious illness in infants;. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; A single dose of 150.
from www.vrogue.co
Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. A single dose of 150. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. A single dose of 150. “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Identify hepatotoxicity concerns and serious illness in infants;.
Paracetamol Induced Hepatotoxicity Intechopen vrogue.co
Paracetamol Hepatotoxicity In Pediatrics This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. A single dose of 150. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. A single dose of 150. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. Identify hepatotoxicity concerns and serious illness in infants;.
From www.researchgate.net
Histopathology reports of liver paracetamol induced hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics A single dose of 150. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. This is the first retrospective study to assess prognostic risk factors in. Paracetamol Hepatotoxicity In Pediatrics.
From www.researchgate.net
(PDF) Extemporaneous Compounding and Stability Evaluation of Paracetamol Hepatotoxicity In Pediatrics A single dose of 150. A single dose of 150. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. Young children appear less at risk. Paracetamol Hepatotoxicity In Pediatrics.
From www.vrogue.co
Paracetamol Induced Hepatotoxicity Intechopen vrogue.co Paracetamol Hepatotoxicity In Pediatrics Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Identify hepatotoxicity concerns and serious illness in infants;. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; A single dose of 150. “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. This is the first. Paracetamol Hepatotoxicity In Pediatrics.
From bpspubs.onlinelibrary.wiley.com
Mitophagy and endoplasmic reticulum‐phagy accelerated by a p62 ZZ Paracetamol Hepatotoxicity In Pediatrics Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. A single dose of 150. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western. Paracetamol Hepatotoxicity In Pediatrics.
From www.semanticscholar.org
[PDF] Acetaminophenrelated hepatotoxicity. Semantic Scholar Paracetamol Hepatotoxicity In Pediatrics Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; A single dose of 150. A single dose of 150. Identify hepatotoxicity concerns and serious illness in infants;. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in. Paracetamol Hepatotoxicity In Pediatrics.
From www.mdpi.com
Medicina Free FullText Risk Factors for Hepatotoxicity Due to Paracetamol Hepatotoxicity In Pediatrics Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. Identify hepatotoxicity concerns and serious illness in infants;. A single dose of. Paracetamol Hepatotoxicity In Pediatrics.
From www.slideshare.net
Paracetamol hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics A single dose of 150. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. A single dose of 150. Identify hepatotoxicity concerns. Paracetamol Hepatotoxicity In Pediatrics.
From adc.bmj.com
Paracetamol induced hepatotoxicity Archives of Disease in Childhood Paracetamol Hepatotoxicity In Pediatrics In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. A single dose of 150. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. Young children appear less at risk of. Paracetamol Hepatotoxicity In Pediatrics.
From adc.bmj.com
Paracetamol induced hepatotoxicity Archives of Disease in Childhood Paracetamol Hepatotoxicity In Pediatrics A single dose of 150. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. A single dose of 150. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Understand. Paracetamol Hepatotoxicity In Pediatrics.
From www.mdpi.com
IJMS Free FullText Myristica fragrans Kernels Prevent Paracetamol Paracetamol Hepatotoxicity In Pediatrics Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. A single dose of 150. “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. Identify hepatotoxicity concerns and serious illness in infants;. This is the first retrospective study to assess prognostic risk factors in children with significant. Paracetamol Hepatotoxicity In Pediatrics.
From www.vrogue.co
Paracetamol Induced Hepatotoxicity Intechopen vrogue.co Paracetamol Hepatotoxicity In Pediatrics A single dose of 150. Identify hepatotoxicity concerns and serious illness in infants;. “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for. Paracetamol Hepatotoxicity In Pediatrics.
From www.slideshare.net
Paracetamol hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics A single dose of 150. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Identify hepatotoxicity concerns and serious illness in infants;. In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. Young children appear less at risk of hepatotoxicity due to an increased. Paracetamol Hepatotoxicity In Pediatrics.
From www.youtube.com
Mechanism of Paracetamol (Acetaminophen) Hepatotoxicity. Pathogenesis Paracetamol Hepatotoxicity In Pediatrics A single dose of 150. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. This is the first retrospective study to assess prognostic risk factors in children. Paracetamol Hepatotoxicity In Pediatrics.
From www.researchgate.net
(PDF) Paracetamol Hepatotoxicity How to Prevent Paracetamol Hepatotoxicity In Pediatrics In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. Identify hepatotoxicity concerns and serious illness in infants;. A single dose of 150. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. Young children appear less. Paracetamol Hepatotoxicity In Pediatrics.
From www.researchgate.net
(PDF) Acute and chronic paracetamol overdose in paediatric population Paracetamol Hepatotoxicity In Pediatrics A single dose of 150. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. A single dose of 150. Identify hepatotoxicity concerns and serious illness in infants;. This is the first retrospective study to assess prognostic risk factors in children with significant. Paracetamol Hepatotoxicity In Pediatrics.
From www.slideshare.net
Paracetamol hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Identify hepatotoxicity concerns and serious illness in infants;. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to. Paracetamol Hepatotoxicity In Pediatrics.
From www.researchgate.net
Metabolites protect against acetaminopheninduced hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics Identify hepatotoxicity concerns and serious illness in infants;. A single dose of 150. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. A single dose of 150. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Understand the absorption, distribution, metabolism. Paracetamol Hepatotoxicity In Pediatrics.
From pdfprof.com
synthèse paracétamol protocole Paracetamol Hepatotoxicity In Pediatrics Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. A single dose of 150. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with. Paracetamol Hepatotoxicity In Pediatrics.
From www.animalia-life.club
Acetaminophen Toxicity Mechanism Paracetamol Hepatotoxicity In Pediatrics A single dose of 150. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; Identify hepatotoxicity concerns and serious illness in infants;. A single dose of 150. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Young children appear less at risk of hepatotoxicity due to an. Paracetamol Hepatotoxicity In Pediatrics.
From www.xiahepublishing.com
AcetaminophenInduced Hepatotoxicity a Comprehensive Update Paracetamol Hepatotoxicity In Pediatrics Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; A single dose of 150. In addition, repeated supratherapeutic doses can cause hepatotoxicity. Paracetamol Hepatotoxicity In Pediatrics.
From www.bmj.com
Management of paracetamol poisoning The BMJ Paracetamol Hepatotoxicity In Pediatrics Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. A single dose of 150. Identify hepatotoxicity concerns and serious illness in infants;. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because. Paracetamol Hepatotoxicity In Pediatrics.
From www.semanticscholar.org
Table 1 from Determinants of hepatotoxicity after repeated Paracetamol Hepatotoxicity In Pediatrics Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. A single dose of 150. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. In addition, repeated supratherapeutic doses can cause. Paracetamol Hepatotoxicity In Pediatrics.
From www.researchgate.net
Paracetamolinduced hepatotoxicity assessment of silymarin and prepared Paracetamol Hepatotoxicity In Pediatrics Identify hepatotoxicity concerns and serious illness in infants;. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; A single dose of 150. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Young children appear. Paracetamol Hepatotoxicity In Pediatrics.
From www.semanticscholar.org
Figure 1 from Paracetamol poisoning in children and hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. A single dose of 150. Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; A single dose of 150. In addition, repeated supratherapeutic doses can. Paracetamol Hepatotoxicity In Pediatrics.
From www.intechopen.com
ParacetamolInduced Hepatotoxicity IntechOpen Paracetamol Hepatotoxicity In Pediatrics In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. A single dose of 150. A single dose of 150. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. This is the. Paracetamol Hepatotoxicity In Pediatrics.
From www.slideshare.net
Paracetamol hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. This is. Paracetamol Hepatotoxicity In Pediatrics.
From www.slideshare.net
Paracetamol hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics Identify hepatotoxicity concerns and serious illness in infants;. A single dose of 150. “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. A single dose of 150. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Young children appear less at risk of hepatotoxicity due to. Paracetamol Hepatotoxicity In Pediatrics.
From www.slideshare.net
Paracetamol hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. A single dose of 150. Identify hepatotoxicity concerns and serious illness in infants;. This is the first retrospective. Paracetamol Hepatotoxicity In Pediatrics.
From www.youtube.com
Paracetamol Pediatric Elixir how to prepare Paracetamol pediatric Paracetamol Hepatotoxicity In Pediatrics Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. A single dose of 150. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. Identify hepatotoxicity concerns and serious illness in infants;. This is the first retrospective. Paracetamol Hepatotoxicity In Pediatrics.
From www.vrogue.co
Paracetamol Induced Hepatotoxicity Intechopen vrogue.co Paracetamol Hepatotoxicity In Pediatrics This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; Identify hepatotoxicity concerns and serious illness in infants;. A single dose of 150. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol.. Paracetamol Hepatotoxicity In Pediatrics.
From www.mdpi.com
Antioxidants Free FullText Sanghuangporus sanghuang Mycelium Paracetamol Hepatotoxicity In Pediatrics This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. Understand the absorption, distribution, metabolism and elimination of paracetamol. Paracetamol Hepatotoxicity In Pediatrics.
From www.researchgate.net
Paracetamol induced hepatotoxicity. (A) Paracetamol induced Paracetamol Hepatotoxicity In Pediatrics Identify hepatotoxicity concerns and serious illness in infants;. “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. A single dose of 150. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for. Paracetamol Hepatotoxicity In Pediatrics.
From www.consultant360.com
Acetaminophen Toxicity in Children Diagnosis, Clinical Assessment, and Paracetamol Hepatotoxicity In Pediatrics “relatively free of adverse reactions” without citation and 9 citations provided for the statement that hepatotoxicity. A single dose of 150. Understand the absorption, distribution, metabolism and elimination of paracetamol in infants; This is the first retrospective study to assess prognostic risk factors in children with significant paracetamol hepatotoxicity and to consider. Young children appear less at risk of hepatotoxicity. Paracetamol Hepatotoxicity In Pediatrics.
From www.slideshare.net
Paracetamol hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics Acetaminophen (apap) overdose is the clinically most relevant drug hepatotoxicity in western countries, and, because of translational. A single dose of 150. In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. “relatively free of adverse reactions” without citation. Paracetamol Hepatotoxicity In Pediatrics.
From www.slideshare.net
Paracetamol hepatotoxicity Paracetamol Hepatotoxicity In Pediatrics Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. Young children appear less at risk of hepatotoxicity due to an increased metabolic capacity for paracetamol. In addition, repeated supratherapeutic doses can cause hepatotoxicity in children with certain risk factors, including. A single dose of 150. A single dose of 150. Identify hepatotoxicity concerns. Paracetamol Hepatotoxicity In Pediatrics.