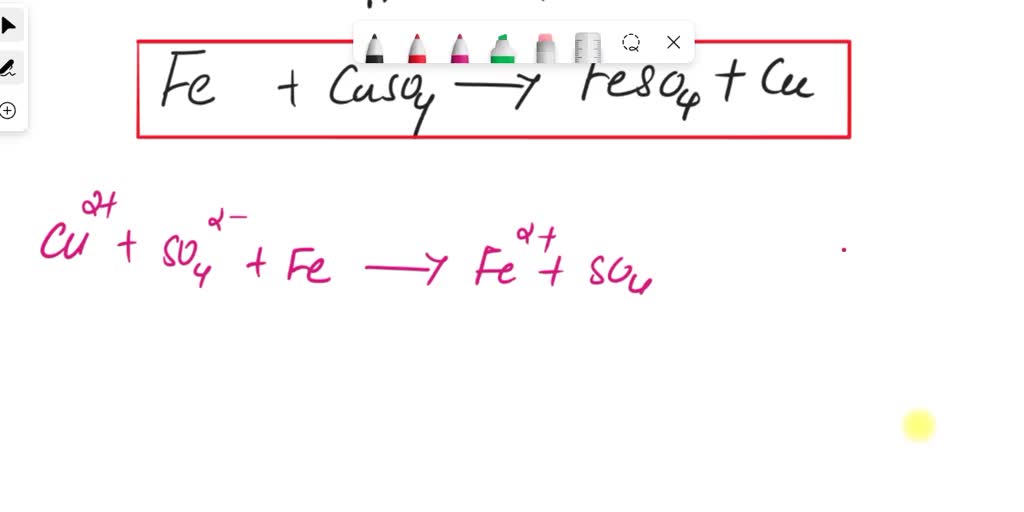Steel Wool Word Equation . The word and symbol equations are: Identify oxygen as a reactant, and water and carbon. Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. The explanation for this goes beyond what is required at key stage 3 but it is summarised. Write the equation for the combustion of steel wool (iron). Magnesium + oxygen → magnesium oxide. Dioxide as products, in combustion of organic materials; Steel wool consists mostly of iron. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. We can write a general word equation for reactions in which a metal reacts with oxygen: When the steel wool is placed in 100% oxygen the. Metal + oxygen → metal oxide. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Burning steel wool is a chemical reaction. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five.
from www.numerade.com
When the steel wool is placed in 100% oxygen the. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. Write the equation for the combustion of steel wool (iron). The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Steel wool (iron) will burn in air quite gently, since air is about 20% (by volume) oxygen. The word and symbol equations are: Magnesium + oxygen → magnesium oxide. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. As the reaction between iron and oxygen produces iron oxide, the mass increases. Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in.
SOLVED reacting steel wool (iron) with copper (ii) sulfate what is
Steel Wool Word Equation Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. We can write a general word equation for reactions in which a metal reacts with oxygen: The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Magnesium + oxygen → magnesium oxide. Metal + oxygen → metal oxide. Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. Burning steel wool is a chemical reaction. Dioxide as products, in combustion of organic materials; Identify oxygen as a reactant, and water and carbon. Steel wool consists mostly of iron. Steel wool (iron) will burn in air quite gently, since air is about 20% (by volume) oxygen. The word and symbol equations are: The explanation for this goes beyond what is required at key stage 3 but it is summarised. Write the equation for the combustion of steel wool (iron).
From www.housedigest.com
The Most Important Place To Use Steel Wool That You're Probably Missing Steel Wool Word Equation Write the equation for the combustion of steel wool (iron). The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. When the steel wool is placed in 100% oxygen the. As the reaction between iron and oxygen produces iron oxide, the mass increases. Magnesium + oxygen → magnesium. Steel Wool Word Equation.
From www.numerade.com
SOLVED MuLmpLE choicequeston The main reaction occurring now that the Steel Wool Word Equation When the steel wool is placed in 100% oxygen the. Burning steel wool is a chemical reaction. Dioxide as products, in combustion of organic materials; Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. Metal + oxygen → metal oxide. Identify oxygen as a reactant, and water and carbon. Steel wool (iron) will burn in. Steel Wool Word Equation.
From www.upstartmag.co.nz
Steel Wool Chemical Reaction Experiment for Kids — Upstart Magazine Steel Wool Word Equation Write the equation for the combustion of steel wool (iron). Magnesium + oxygen → magnesium oxide. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Steel wool (iron) will burn in air quite gently, since air is about 20% (by volume) oxygen. Burning steel wool is a chemical reaction. When the steel wool is placed in 100% oxygen the. The explanation. Steel Wool Word Equation.
From www.youtube.com
Copper sulfate and steel wool YouTube Steel Wool Word Equation The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. Magnesium + oxygen → magnesium oxide. Burning steel wool is a chemical reaction. Dioxide as products, in combustion of organic materials; Identify oxygen as a reactant, and water and carbon. Steel wool consists mostly. Steel Wool Word Equation.
From lightpaintingphotography.com
Unique Steel Wool Fire Photography Technique, Light Painting VLOG 47 Steel Wool Word Equation Identify oxygen as a reactant, and water and carbon. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). The explanation for this goes beyond what is required at key stage 3 but it is summarised. Metal + oxygen → metal oxide.. Steel Wool Word Equation.
From www.slideshare.net
Steel Wool and ETextiles Steel Wool Word Equation Identify oxygen as a reactant, and water and carbon. The word and symbol equations are: Steel wool consists mostly of iron. Steel wool (iron) will burn in air quite gently, since air is about 20% (by volume) oxygen. We can write a general word equation for reactions in which a metal reacts with oxygen: The combustion of iron produces iron(iii). Steel Wool Word Equation.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Steel Wool Word Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Metal + oxygen → metal oxide. Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. Steel wool consists mostly of iron. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five.. Steel Wool Word Equation.
From ar.inspiredpencil.com
Structural Formula Of Iron Oxide Steel Wool Word Equation The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. We can write a general word equation for reactions in which a metal reacts with oxygen: Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. Students may want to. Steel Wool Word Equation.
From mugeek.vidalondon.net
Chemical Makeup Of Steel Wool Mugeek Vidalondon Steel Wool Word Equation Magnesium + oxygen → magnesium oxide. When the steel wool is placed in 100% oxygen the. The word and symbol equations are: The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). We can write a general word equation for reactions in which a metal reacts with oxygen: Metal + oxygen → metal oxide. Steel wool (iron) will burn in air quite. Steel Wool Word Equation.
From thewonderofscience.com
Burning Steel Wool — The Wonder of Science Steel Wool Word Equation Write the equation for the combustion of steel wool (iron). Magnesium + oxygen → magnesium oxide. Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. We can write a general word equation for reactions in which a metal reacts with oxygen: The chemical equation for this reaction is 2h 2 (g) + o 2 →. Steel Wool Word Equation.
From teachingscience.us
Combustion Reaction Burning Steel Wool Teaching Science with Lynda R Steel Wool Word Equation Write the equation for the combustion of steel wool (iron). Magnesium + oxygen → magnesium oxide. The explanation for this goes beyond what is required at key stage 3 but it is summarised. Dioxide as products, in combustion of organic materials; Burning steel wool is a chemical reaction. The chemical equation for this reaction is 2h 2 (g) + o. Steel Wool Word Equation.
From www.numerade.com
SOLVED In this reaction, iron (steel wool) is combining with oxygen to Steel Wool Word Equation Metal + oxygen → metal oxide. Steel wool consists mostly of iron. The explanation for this goes beyond what is required at key stage 3 but it is summarised. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). We can write a general word equation for reactions in which a metal reacts with oxygen: When the steel wool is placed in. Steel Wool Word Equation.
From www.youtube.com
Burning Steel Wool Does the mass increase or decrease? YouTube Steel Wool Word Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Burning steel wool is a chemical reaction. Metal + oxygen → metal oxide. Write the equation for the combustion of steel wool (iron). As the reaction between iron and oxygen produces iron oxide, the mass increases. Identify oxygen as a reactant, and water and carbon. We can write a general word equation. Steel Wool Word Equation.
From littlealchemy2.gambledude.com
steel wool Little Alchemy 2 Cheats Steel Wool Word Equation Burning steel wool is a chemical reaction. The explanation for this goes beyond what is required at key stage 3 but it is summarised. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. Dioxide as products, in combustion of organic materials; We can. Steel Wool Word Equation.
From www.chegg.com
Solved II. Reaction of Iron (steel wool) with copper (II) Steel Wool Word Equation When the steel wool is placed in 100% oxygen the. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). The explanation for this goes beyond what is required at key stage 3 but it is summarised. Metal + oxygen → metal oxide. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will. Steel Wool Word Equation.
From www.chegg.com
Solved 1. Burned steel wool (pure Fe) a dark comp Steel Wool Word Equation Burning steel wool is a chemical reaction. Steel wool consists mostly of iron. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. Magnesium. Steel Wool Word Equation.
From www.livescience.com
Here's How Steel Wool Burns (and Why It Looks Like the Death of Krypton Steel Wool Word Equation The word and symbol equations are: Burning steel wool is a chemical reaction. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. Magnesium + oxygen → magnesium oxide. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). The explanation for this goes beyond what. Steel Wool Word Equation.
From www.numerade.com
SOLVED reacting steel wool (iron) with copper (ii) sulfate what is Steel Wool Word Equation Steel wool (iron) will burn in air quite gently, since air is about 20% (by volume) oxygen. The word and symbol equations are: Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. Dioxide as products, in combustion of organic materials; When the steel wool is placed in 100% oxygen. Steel Wool Word Equation.
From www.slideshare.net
Replacement reactions Steel Wool Word Equation Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. We can write a general word equation for reactions in which a metal reacts with oxygen: The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. Dioxide as products, in. Steel Wool Word Equation.
From studylibraryintroit.z14.web.core.windows.net
Formula For Steel Wool Steel Wool Word Equation Steel wool consists mostly of iron. Dioxide as products, in combustion of organic materials; Burning steel wool is a chemical reaction. Metal + oxygen → metal oxide. Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will. Steel Wool Word Equation.
From mugeek.vidalondon.net
Chemical Makeup Of Steel Wool Mugeek Vidalondon Steel Wool Word Equation Write the equation for the combustion of steel wool (iron). When the steel wool is placed in 100% oxygen the. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. Steel wool (iron) will burn in air quite gently, since air is about 20% (by volume) oxygen. Students. Steel Wool Word Equation.
From www.familyhandyman.com
Steel Wool Comes in 8 Different Grades—Here's How to Use Each One Steel Wool Word Equation When the steel wool is placed in 100% oxygen the. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Identify oxygen as a reactant, and water and carbon. Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. Steel wool (iron) will burn in air quite gently, since air is about 20% (by volume) oxygen. The chemical. Steel Wool Word Equation.
From hxegskbdu.blob.core.windows.net
Burning Steel Wool Equation at Josephine Luken blog Steel Wool Word Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). The word and symbol equations are: Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. Steel wool consists mostly of iron.. Steel Wool Word Equation.
From www.youtube.com
How to ignite steel wool with a battery ("Steel wool" experiment) YouTube Steel Wool Word Equation The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Steel wool (iron) will burn in air quite gently, since air is about 20% (by volume) oxygen. Steel wool consists mostly of iron. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. Write the equation for the combustion of steel wool. Steel Wool Word Equation.
From alchemistcheats.com
Little Alchemy 2 Cheats How to Make Steel Wool Steel Wool Word Equation Dioxide as products, in combustion of organic materials; The explanation for this goes beyond what is required at key stage 3 but it is summarised. Metal + oxygen → metal oxide. As the reaction between iron and oxygen produces iron oxide, the mass increases. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). We can write a general word equation for. Steel Wool Word Equation.
From www.liveworksheets.com
Steel Wool Experiment worksheet Live Worksheets Steel Wool Word Equation Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not.. Steel Wool Word Equation.
From www.researchgate.net
(PDF) Iron acetate solution prepared from steel wool and vinegar for Steel Wool Word Equation The word and symbol equations are: As the reaction between iron and oxygen produces iron oxide, the mass increases. Metal + oxygen → metal oxide. Burning steel wool is a chemical reaction. Magnesium + oxygen → magnesium oxide. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. Identify oxygen. Steel Wool Word Equation.
From hospecoonline.ph
Stainless Steel Wool Kitchen Dish Ball (1pack) Hospeco Steel Wool Word Equation Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. Write the equation for the combustion of steel wool (iron). The explanation for this goes beyond what is required at key stage 3 but it is summarised. Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used. Steel Wool Word Equation.
From oneclass.com
OneClass Write a balanced equation for the reaction of the steel wool Steel Wool Word Equation We can write a general word equation for reactions in which a metal reacts with oxygen: Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. The combustion of iron produces iron(iii) oxide, \(\ce{fe2o3}\). Steel wool consists mostly of iron. Write the equation for the combustion of steel wool (iron). The word and symbol equations are:. Steel Wool Word Equation.
From slidetodoc.com
Types of Reactions 1 Synthesis reactions 2 Steel Wool Word Equation As the reaction between iron and oxygen produces iron oxide, the mass increases. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. Mg. Steel Wool Word Equation.
From www.numerade.com
SOLVED Explain the differences in the observations for the bottles Steel Wool Word Equation Identify oxygen as a reactant, and water and carbon. Metal + oxygen → metal oxide. We can write a general word equation for reactions in which a metal reacts with oxygen: As the reaction between iron and oxygen produces iron oxide, the mass increases. The explanation for this goes beyond what is required at key stage 3 but it is. Steel Wool Word Equation.
From www.youtube.com
February 01 2012 period3 1 YouTube Steel Wool Word Equation Identify oxygen as a reactant, and water and carbon. Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. When the steel wool is placed in 100% oxygen the. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. The. Steel Wool Word Equation.
From www.familyhandyman.com
Steel Wool Comes in 8 Different Grades—Here's How to Use Each One Steel Wool Word Equation Metal + oxygen → metal oxide. Identify oxygen as a reactant, and water and carbon. Mg (s) + o₂ (g) → mgo (s) state symbol an abbreviation used in. The explanation for this goes beyond what is required at key stage 3 but it is summarised. When the steel wool is placed in 100% oxygen the. Dioxide as products, in. Steel Wool Word Equation.
From materialmcgheepearter.z21.web.core.windows.net
Steel Wool Chemical Formula Steel Wool Word Equation When the steel wool is placed in 100% oxygen the. The chemical equation for this reaction is 2h 2 (g) + o 2 → 2h 2 o (g) i use this reaction as one of five. As the reaction between iron and oxygen produces iron oxide, the mass increases. Magnesium + oxygen → magnesium oxide. The combustion of iron produces. Steel Wool Word Equation.
From www.youtube.com
Steel Wool and Vinegar Experiment YouTube Steel Wool Word Equation Steel wool consists mostly of iron. When the steel wool is placed in 100% oxygen the. Students may want to know why iron wool combusts, but bigger bits of iron such as iron nails will not. As the reaction between iron and oxygen produces iron oxide, the mass increases. Write the equation for the combustion of steel wool (iron). Steel. Steel Wool Word Equation.