If Chlorine Gains Valence Electrons Then It Will Become . either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. most nonmetals become anions when they make ionic compounds. When forming ions, elements typically gain or lose. chlorine is a halogen. so for chlorine, if we think about how chlorine reacts, chlorine has. Metals lose electrons, nonmetals gain electrons. In general, metals will lose electrons to become a positive cation. if sodium metal and chlorine gas mix under the right conditions, they will form salt. Halogens have seven valence electrons so chlorine's valence electrons would look like this. The sodium loses an electron, and the chlorine. most nonmetals become anions when they make ionic compounds. A neutral chlorine atom has seven electrons in its outermost. A neutral chlorine atom has seven electrons in its outermost.
from socratic.org
chlorine is a halogen. When forming ions, elements typically gain or lose. Metals lose electrons, nonmetals gain electrons. most nonmetals become anions when they make ionic compounds. so for chlorine, if we think about how chlorine reacts, chlorine has. Halogens have seven valence electrons so chlorine's valence electrons would look like this. A neutral chlorine atom has seven electrons in its outermost. most nonmetals become anions when they make ionic compounds. The sodium loses an electron, and the chlorine. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons.
Chlorine combined with two negative atom or 1 positive and other
If Chlorine Gains Valence Electrons Then It Will Become most nonmetals become anions when they make ionic compounds. A neutral chlorine atom has seven electrons in its outermost. A neutral chlorine atom has seven electrons in its outermost. Halogens have seven valence electrons so chlorine's valence electrons would look like this. When forming ions, elements typically gain or lose. The sodium loses an electron, and the chlorine. chlorine is a halogen. if sodium metal and chlorine gas mix under the right conditions, they will form salt. most nonmetals become anions when they make ionic compounds. most nonmetals become anions when they make ionic compounds. Metals lose electrons, nonmetals gain electrons. so for chlorine, if we think about how chlorine reacts, chlorine has. In general, metals will lose electrons to become a positive cation. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons.
From www.chemtopper.com
How to draw Lewis Dot Structure Online Chemistry Tutor If Chlorine Gains Valence Electrons Then It Will Become When forming ions, elements typically gain or lose. if sodium metal and chlorine gas mix under the right conditions, they will form salt. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. The sodium loses an electron, and the chlorine. Metals lose electrons,. If Chlorine Gains Valence Electrons Then It Will Become.
From slideplayer.com
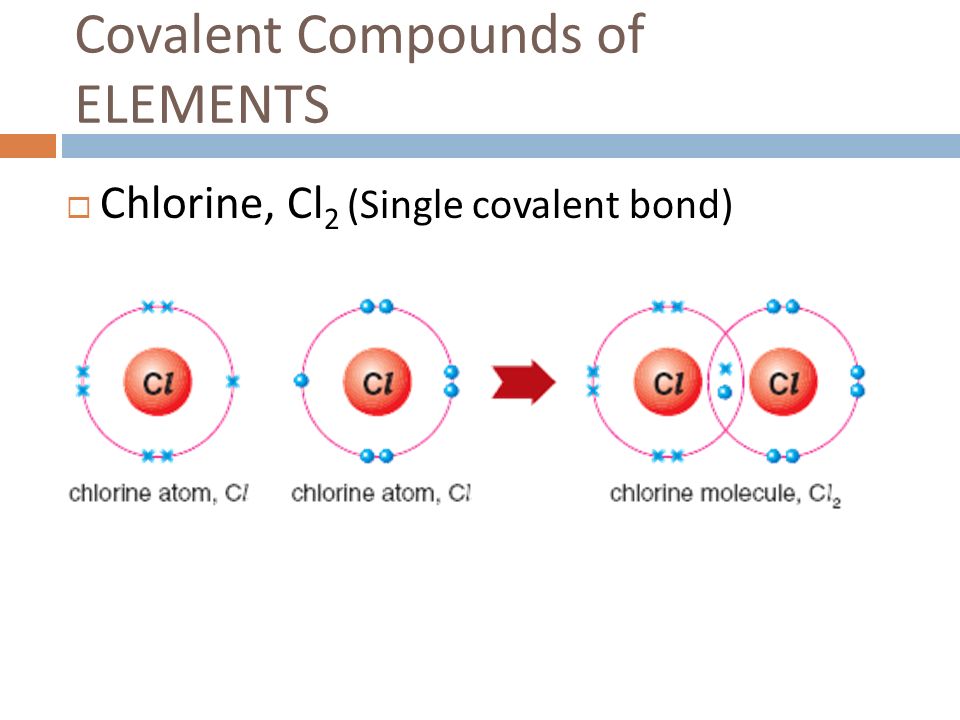
Chapter 4 Formation of Compounds ppt download If Chlorine Gains Valence Electrons Then It Will Become In general, metals will lose electrons to become a positive cation. When forming ions, elements typically gain or lose. A neutral chlorine atom has seven electrons in its outermost. most nonmetals become anions when they make ionic compounds. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. If Chlorine Gains Valence Electrons Then It Will Become.
From courses.lumenlearning.com
2.2 Bonding and Lattices Physical Geology If Chlorine Gains Valence Electrons Then It Will Become chlorine is a halogen. so for chlorine, if we think about how chlorine reacts, chlorine has. When forming ions, elements typically gain or lose. The sodium loses an electron, and the chlorine. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. Metals. If Chlorine Gains Valence Electrons Then It Will Become.
From www.labxchange.org
LabXchange If Chlorine Gains Valence Electrons Then It Will Become most nonmetals become anions when they make ionic compounds. Metals lose electrons, nonmetals gain electrons. Halogens have seven valence electrons so chlorine's valence electrons would look like this. A neutral chlorine atom has seven electrons in its outermost. most nonmetals become anions when they make ionic compounds. In general, metals will lose electrons to become a positive cation.. If Chlorine Gains Valence Electrons Then It Will Become.
From www.youtube.com
How to Find the Valence Electrons for Chlorine (Cl) YouTube If Chlorine Gains Valence Electrons Then It Will Become The sodium loses an electron, and the chlorine. most nonmetals become anions when they make ionic compounds. so for chlorine, if we think about how chlorine reacts, chlorine has. A neutral chlorine atom has seven electrons in its outermost. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged. If Chlorine Gains Valence Electrons Then It Will Become.
From www.nagwa.com
Question Video Recalling the Species Formed When a Chlorine Atom Gains If Chlorine Gains Valence Electrons Then It Will Become either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. In general, metals will lose electrons to become a positive cation. if sodium metal and chlorine gas mix under the right conditions, they will form salt. A neutral chlorine atom has seven electrons in. If Chlorine Gains Valence Electrons Then It Will Become.
From www.vrogue.co
How Many Electrons And Protons Does An Atom Have 2023 vrogue.co If Chlorine Gains Valence Electrons Then It Will Become A neutral chlorine atom has seven electrons in its outermost. chlorine is a halogen. When forming ions, elements typically gain or lose. A neutral chlorine atom has seven electrons in its outermost. Metals lose electrons, nonmetals gain electrons. most nonmetals become anions when they make ionic compounds. The sodium loses an electron, and the chlorine. Halogens have seven. If Chlorine Gains Valence Electrons Then It Will Become.
From ar.inspiredpencil.com
Periodic Table Valence Electrons If Chlorine Gains Valence Electrons Then It Will Become A neutral chlorine atom has seven electrons in its outermost. In general, metals will lose electrons to become a positive cation. A neutral chlorine atom has seven electrons in its outermost. When forming ions, elements typically gain or lose. The sodium loses an electron, and the chlorine. either atoms gain enough electrons to have eight electrons in the valence. If Chlorine Gains Valence Electrons Then It Will Become.
From topblogtenz.com
How to find Valence electrons Various method and Examples If Chlorine Gains Valence Electrons Then It Will Become most nonmetals become anions when they make ionic compounds. so for chlorine, if we think about how chlorine reacts, chlorine has. Halogens have seven valence electrons so chlorine's valence electrons would look like this. In general, metals will lose electrons to become a positive cation. A neutral chlorine atom has seven electrons in its outermost. A neutral chlorine. If Chlorine Gains Valence Electrons Then It Will Become.
From slideplayer.com
Chemistry 10 Ions (Cations & Anions) Bohr Diagrams Lewis Dot Diagrams If Chlorine Gains Valence Electrons Then It Will Become if sodium metal and chlorine gas mix under the right conditions, they will form salt. chlorine is a halogen. A neutral chlorine atom has seven electrons in its outermost. The sodium loses an electron, and the chlorine. Halogens have seven valence electrons so chlorine's valence electrons would look like this. In general, metals will lose electrons to become. If Chlorine Gains Valence Electrons Then It Will Become.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science If Chlorine Gains Valence Electrons Then It Will Become When forming ions, elements typically gain or lose. so for chlorine, if we think about how chlorine reacts, chlorine has. In general, metals will lose electrons to become a positive cation. most nonmetals become anions when they make ionic compounds. if sodium metal and chlorine gas mix under the right conditions, they will form salt. A neutral. If Chlorine Gains Valence Electrons Then It Will Become.
From snareabear.blogspot.com
Valence Electrons Of Chlorine chlorine Electron configuration, Atom If Chlorine Gains Valence Electrons Then It Will Become most nonmetals become anions when they make ionic compounds. most nonmetals become anions when they make ionic compounds. Halogens have seven valence electrons so chlorine's valence electrons would look like this. so for chlorine, if we think about how chlorine reacts, chlorine has. The sodium loses an electron, and the chlorine. A neutral chlorine atom has seven. If Chlorine Gains Valence Electrons Then It Will Become.
From www.myxxgirl.com
Chlorine Orbital Diagram Electron Configuration And Valence Electrons If Chlorine Gains Valence Electrons Then It Will Become The sodium loses an electron, and the chlorine. so for chlorine, if we think about how chlorine reacts, chlorine has. In general, metals will lose electrons to become a positive cation. most nonmetals become anions when they make ionic compounds. chlorine is a halogen. Halogens have seven valence electrons so chlorine's valence electrons would look like this.. If Chlorine Gains Valence Electrons Then It Will Become.
From ratuwedding99.blogspot.com
How Many Valence Electrons Are In Chlorine ratu wedding If Chlorine Gains Valence Electrons Then It Will Become In general, metals will lose electrons to become a positive cation. A neutral chlorine atom has seven electrons in its outermost. chlorine is a halogen. Halogens have seven valence electrons so chlorine's valence electrons would look like this. if sodium metal and chlorine gas mix under the right conditions, they will form salt. so for chlorine, if. If Chlorine Gains Valence Electrons Then It Will Become.
From the-electron-2020.blogspot.com
The Electron 2020 Give The Electron Configuration Of Chlorine If Chlorine Gains Valence Electrons Then It Will Become chlorine is a halogen. When forming ions, elements typically gain or lose. Halogens have seven valence electrons so chlorine's valence electrons would look like this. In general, metals will lose electrons to become a positive cation. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose. If Chlorine Gains Valence Electrons Then It Will Become.
From socratic.org
Question 587a9 Socratic If Chlorine Gains Valence Electrons Then It Will Become chlorine is a halogen. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. A neutral chlorine atom has seven electrons in its outermost. most nonmetals become anions when they make ionic compounds. When forming ions, elements typically gain or lose. if. If Chlorine Gains Valence Electrons Then It Will Become.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons If Chlorine Gains Valence Electrons Then It Will Become A neutral chlorine atom has seven electrons in its outermost. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. chlorine is a halogen. A neutral chlorine atom has seven electrons in its outermost. Halogens have seven valence electrons so chlorine's valence electrons would. If Chlorine Gains Valence Electrons Then It Will Become.
From www.numerade.com
SOLVEDWhen chlorine gains an electron to a chloride ion with a If Chlorine Gains Valence Electrons Then It Will Become A neutral chlorine atom has seven electrons in its outermost. Halogens have seven valence electrons so chlorine's valence electrons would look like this. Metals lose electrons, nonmetals gain electrons. if sodium metal and chlorine gas mix under the right conditions, they will form salt. chlorine is a halogen. so for chlorine, if we think about how chlorine. If Chlorine Gains Valence Electrons Then It Will Become.
From www.periodictableprintable.com
How To Find Number Of Valence Electrons On Periodic Table Periodic If Chlorine Gains Valence Electrons Then It Will Become so for chlorine, if we think about how chlorine reacts, chlorine has. Halogens have seven valence electrons so chlorine's valence electrons would look like this. A neutral chlorine atom has seven electrons in its outermost. In general, metals will lose electrons to become a positive cation. most nonmetals become anions when they make ionic compounds. chlorine is. If Chlorine Gains Valence Electrons Then It Will Become.
From smallbusinessron.web.fc2.com
Topic If Chlorine Gains Valence Electrons Then It Will Become A neutral chlorine atom has seven electrons in its outermost. so for chlorine, if we think about how chlorine reacts, chlorine has. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. When forming ions, elements typically gain or lose. Metals lose electrons, nonmetals. If Chlorine Gains Valence Electrons Then It Will Become.
From www.numerade.com
SOLVEDWhen chlorine gains an electron to a chloride ion with a If Chlorine Gains Valence Electrons Then It Will Become When forming ions, elements typically gain or lose. Metals lose electrons, nonmetals gain electrons. Halogens have seven valence electrons so chlorine's valence electrons would look like this. A neutral chlorine atom has seven electrons in its outermost. most nonmetals become anions when they make ionic compounds. A neutral chlorine atom has seven electrons in its outermost. if sodium. If Chlorine Gains Valence Electrons Then It Will Become.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions If Chlorine Gains Valence Electrons Then It Will Become most nonmetals become anions when they make ionic compounds. A neutral chlorine atom has seven electrons in its outermost. The sodium loses an electron, and the chlorine. most nonmetals become anions when they make ionic compounds. chlorine is a halogen. Metals lose electrons, nonmetals gain electrons. if sodium metal and chlorine gas mix under the right. If Chlorine Gains Valence Electrons Then It Will Become.
From courses.lumenlearning.com
3.2 Ions The Basics of General, Organic, and Biological Chemistry If Chlorine Gains Valence Electrons Then It Will Become most nonmetals become anions when they make ionic compounds. if sodium metal and chlorine gas mix under the right conditions, they will form salt. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. chlorine is a halogen. A neutral chlorine atom. If Chlorine Gains Valence Electrons Then It Will Become.
From montessorimuddle.org
subatomic particles Montessori Muddle If Chlorine Gains Valence Electrons Then It Will Become most nonmetals become anions when they make ionic compounds. chlorine is a halogen. The sodium loses an electron, and the chlorine. Halogens have seven valence electrons so chlorine's valence electrons would look like this. Metals lose electrons, nonmetals gain electrons. if sodium metal and chlorine gas mix under the right conditions, they will form salt. In general,. If Chlorine Gains Valence Electrons Then It Will Become.
From www.pngegg.com
Schéma d'électrons de Valence au chlore et à la structure de Lewis, 18 If Chlorine Gains Valence Electrons Then It Will Become A neutral chlorine atom has seven electrons in its outermost. A neutral chlorine atom has seven electrons in its outermost. so for chlorine, if we think about how chlorine reacts, chlorine has. most nonmetals become anions when they make ionic compounds. either atoms gain enough electrons to have eight electrons in the valence shell and become the. If Chlorine Gains Valence Electrons Then It Will Become.
From www.chegg.com
Solved If chlorine gains one electron, how many valence If Chlorine Gains Valence Electrons Then It Will Become so for chlorine, if we think about how chlorine reacts, chlorine has. When forming ions, elements typically gain or lose. chlorine is a halogen. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. Metals lose electrons, nonmetals gain electrons. A neutral chlorine. If Chlorine Gains Valence Electrons Then It Will Become.
From byjus.com
In the electron dot structure, the valence shell electrons are If Chlorine Gains Valence Electrons Then It Will Become most nonmetals become anions when they make ionic compounds. if sodium metal and chlorine gas mix under the right conditions, they will form salt. Metals lose electrons, nonmetals gain electrons. When forming ions, elements typically gain or lose. chlorine is a halogen. The sodium loses an electron, and the chlorine. A neutral chlorine atom has seven electrons. If Chlorine Gains Valence Electrons Then It Will Become.
From valenceelectrons.com
How to Find the Valence Electrons for Chlorine (Cl)? If Chlorine Gains Valence Electrons Then It Will Become so for chlorine, if we think about how chlorine reacts, chlorine has. Metals lose electrons, nonmetals gain electrons. Halogens have seven valence electrons so chlorine's valence electrons would look like this. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. most nonmetals. If Chlorine Gains Valence Electrons Then It Will Become.
From socratic.org
Chlorine combined with two negative atom or 1 positive and other If Chlorine Gains Valence Electrons Then It Will Become most nonmetals become anions when they make ionic compounds. so for chlorine, if we think about how chlorine reacts, chlorine has. if sodium metal and chlorine gas mix under the right conditions, they will form salt. chlorine is a halogen. The sodium loses an electron, and the chlorine. Halogens have seven valence electrons so chlorine's valence. If Chlorine Gains Valence Electrons Then It Will Become.
From byjus.com
Electronic Configuration Distribution of Electrons Chemistry If Chlorine Gains Valence Electrons Then It Will Become most nonmetals become anions when they make ionic compounds. chlorine is a halogen. so for chlorine, if we think about how chlorine reacts, chlorine has. The sodium loses an electron, and the chlorine. In general, metals will lose electrons to become a positive cation. Metals lose electrons, nonmetals gain electrons. Halogens have seven valence electrons so chlorine's. If Chlorine Gains Valence Electrons Then It Will Become.
From valenceelectrons.com
How to Find the Valence Electrons for Chlorine (Cl)? If Chlorine Gains Valence Electrons Then It Will Become The sodium loses an electron, and the chlorine. A neutral chlorine atom has seven electrons in its outermost. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. Metals lose electrons, nonmetals gain electrons. if sodium metal and chlorine gas mix under the right. If Chlorine Gains Valence Electrons Then It Will Become.
From www.groundwatergovernance.org
The Chloride Ion An Atom’s Journey To Negatively Charged If Chlorine Gains Valence Electrons Then It Will Become The sodium loses an electron, and the chlorine. When forming ions, elements typically gain or lose. if sodium metal and chlorine gas mix under the right conditions, they will form salt. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons. Metals lose electrons,. If Chlorine Gains Valence Electrons Then It Will Become.
From www.numerade.com
SOLVED An atom of lithium (Li) forms an ionic bond with an atom of If Chlorine Gains Valence Electrons Then It Will Become When forming ions, elements typically gain or lose. so for chlorine, if we think about how chlorine reacts, chlorine has. if sodium metal and chlorine gas mix under the right conditions, they will form salt. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose. If Chlorine Gains Valence Electrons Then It Will Become.
From www.slideshare.net
10 28 How Many Electrons Do Atoms Gain Lose If Chlorine Gains Valence Electrons Then It Will Become so for chlorine, if we think about how chlorine reacts, chlorine has. Metals lose electrons, nonmetals gain electrons. most nonmetals become anions when they make ionic compounds. A neutral chlorine atom has seven electrons in its outermost. In general, metals will lose electrons to become a positive cation. either atoms gain enough electrons to have eight electrons. If Chlorine Gains Valence Electrons Then It Will Become.
From www.expii.com
Ions — Definition & Overview Expii If Chlorine Gains Valence Electrons Then It Will Become The sodium loses an electron, and the chlorine. so for chlorine, if we think about how chlorine reacts, chlorine has. When forming ions, elements typically gain or lose. most nonmetals become anions when they make ionic compounds. either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. If Chlorine Gains Valence Electrons Then It Will Become.