How Much Does Water Pressure Increase With Temperature . So whilst the water temperature remains at less than 100°c, the. If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. Yes, at constant density, the pressure increases as the temperature does: The change in specific volume for a given change in temperature is not the same at various beginning temperatures. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases.
from www.vrogue.co
The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. So whilst the water temperature remains at less than 100°c, the. If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). The change in specific volume for a given change in temperature is not the same at various beginning temperatures. Yes, at constant density, the pressure increases as the temperature does: As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases.
Pressure Temperature Phase Diagram For Water Learnche vrogue.co
How Much Does Water Pressure Increase With Temperature The change in specific volume for a given change in temperature is not the same at various beginning temperatures. If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. So whilst the water temperature remains at less than 100°c, the. The change in specific volume for a given change in temperature is not the same at various beginning temperatures. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Yes, at constant density, the pressure increases as the temperature does: The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? How Much Does Water Pressure Increase With Temperature As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. The change in specific volume for a given change in temperature is not the same at various beginning temperatures. Yes, at constant density,. How Much Does Water Pressure Increase With Temperature.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 How Much Does Water Pressure Increase With Temperature The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water. How Much Does Water Pressure Increase With Temperature.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning How Much Does Water Pressure Increase With Temperature Yes, at constant density, the pressure increases as the temperature does: The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). As you heat the pressure cooker, the. How Much Does Water Pressure Increase With Temperature.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ How Much Does Water Pressure Increase With Temperature As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. How Much Does Water Pressure Increase With Temperature.
From www.vrogue.co
Pressure Temperature Phase Diagram For Water Learnche vrogue.co How Much Does Water Pressure Increase With Temperature The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). The change in specific volume for a given change in temperature is not the same at various beginning temperatures. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1. How Much Does Water Pressure Increase With Temperature.
From pressbooks.bccampus.ca
13.5 Phase Changes College Physics OpenStax How Much Does Water Pressure Increase With Temperature As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). The change in specific volume for a given change in temperature is not the same at various beginning temperatures. So whilst the. How Much Does Water Pressure Increase With Temperature.
From recipeprojectblog.com
How to Increase Water Pressure in Kitchen Sink? The Recipe Project How Much Does Water Pressure Increase With Temperature So whilst the water temperature remains at less than 100°c, the. Yes, at constant density, the pressure increases as the temperature does: As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which. How Much Does Water Pressure Increase With Temperature.
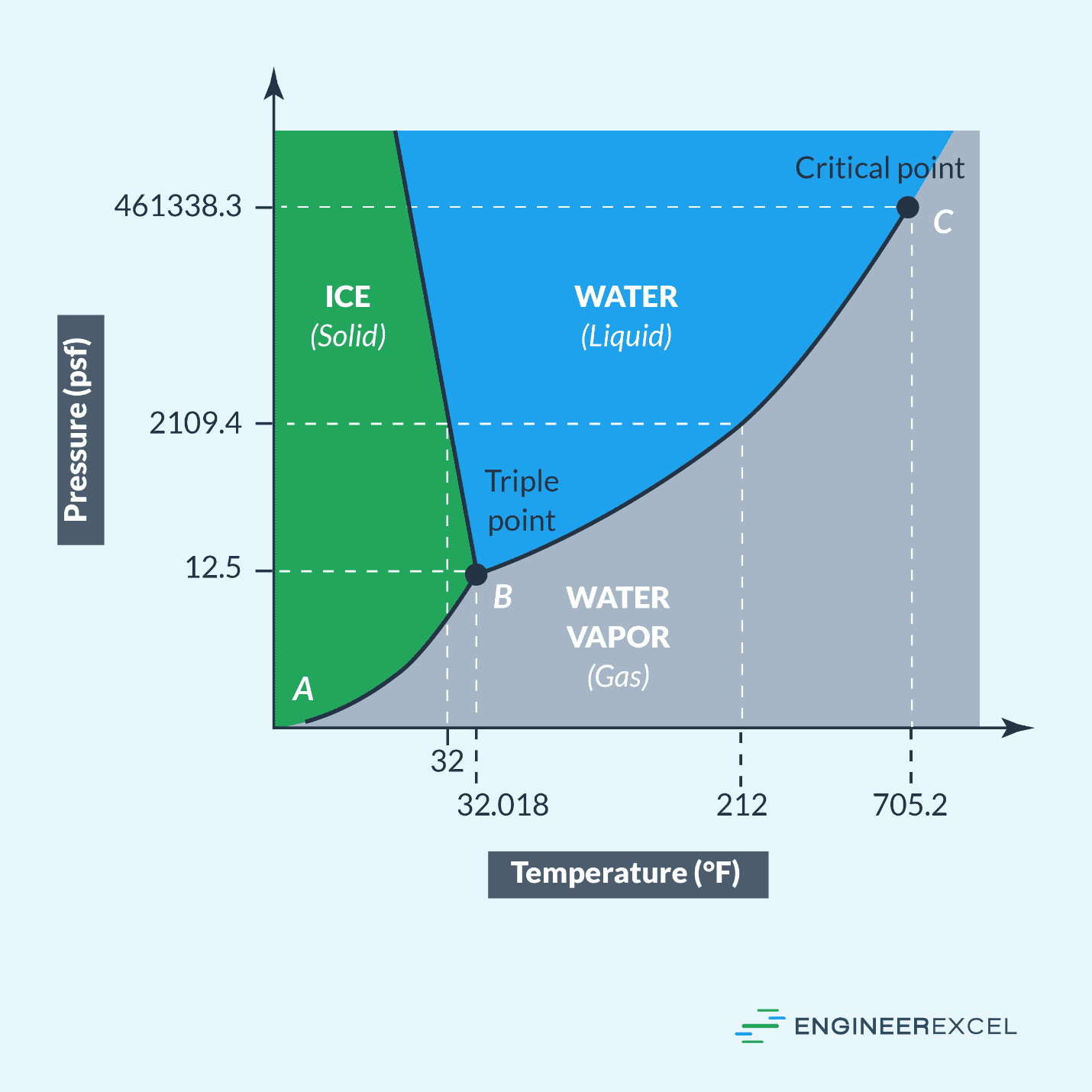
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel How Much Does Water Pressure Increase With Temperature The change in specific volume for a given change in temperature is not the same at various beginning temperatures. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). So whilst the water temperature remains at less than 100°c, the. If you raise the pressure keeping the temperature constant, it'll switch. How Much Does Water Pressure Increase With Temperature.
From www.vedantu.com
What is the relationship between the solubility and the temperature How Much Does Water Pressure Increase With Temperature The change in specific volume for a given change in temperature is not the same at various beginning temperatures. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water. How Much Does Water Pressure Increase With Temperature.
From 4perfectwater.com
How to Increase Water Pressure in Your Home (2020) How Much Does Water Pressure Increase With Temperature If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\).. How Much Does Water Pressure Increase With Temperature.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase How Much Does Water Pressure Increase With Temperature Yes, at constant density, the pressure increases as the temperature does: The change in specific volume for a given change in temperature is not the same at various beginning temperatures. So whilst the water temperature remains at less than 100°c, the. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\).. How Much Does Water Pressure Increase With Temperature.
From sensibledigs.com
Too Much Pressure In Water Heater Causes & Fixes How Much Does Water Pressure Increase With Temperature The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. Yes, at constant density, the pressure increases as the temperature does: If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. As you heat the pressure cooker, the water’s. How Much Does Water Pressure Increase With Temperature.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 How Much Does Water Pressure Increase With Temperature If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. Yes, at constant density, the pressure increases as the temperature does: The relationships among the volume of a gas. How Much Does Water Pressure Increase With Temperature.
From www.slideserve.com
PPT How is pressure calculated? PowerPoint Presentation, free How Much Does Water Pressure Increase With Temperature So whilst the water temperature remains at less than 100°c, the. The change in specific volume for a given change in temperature is not the same at various beginning temperatures. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. The relationships among the volume of a. How Much Does Water Pressure Increase With Temperature.
From www.plumbingsupply.com
Residential Water Pressure Explained How Much Does Water Pressure Increase With Temperature The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). So whilst the water temperature remains at less than 100°c, the. If you raise the pressure keeping the. How Much Does Water Pressure Increase With Temperature.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation How Much Does Water Pressure Increase With Temperature If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. The change in specific volume for a given change in temperature is not the same at various beginning temperatures. As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. The relationships among the. How Much Does Water Pressure Increase With Temperature.
From www.slideserve.com
PPT How is pressure calculated? PowerPoint Presentation, free How Much Does Water Pressure Increase With Temperature So whilst the water temperature remains at less than 100°c, the. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. If you raise the pressure keeping the temperature constant, it'll switch. How Much Does Water Pressure Increase With Temperature.
From www.animalia-life.club
Water Pressure Ocean How Much Does Water Pressure Increase With Temperature As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /.. How Much Does Water Pressure Increase With Temperature.
From www.youtube.com
The Effect of Temperature and Pressure on Solubility Mr C YouTube How Much Does Water Pressure Increase With Temperature As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. So whilst the water temperature remains at less than 100°c, the. If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. Yes, at constant density, the pressure increases as the temperature does: The. How Much Does Water Pressure Increase With Temperature.
From realestateinfoguide.com
10 Ways to Increase Home Water Pressure Real Estate Info Guide How Much Does Water Pressure Increase With Temperature The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases.. How Much Does Water Pressure Increase With Temperature.
From www.researchgate.net
Pressuretemperature phase diagram of water [16]. Download High How Much Does Water Pressure Increase With Temperature The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. Yes, at constant density, the pressure increases as the temperature does: If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. The relationships among the volume of a gas. How Much Does Water Pressure Increase With Temperature.
From www.researchgate.net
PressureTemperature diagram for water. Download Scientific Diagram How Much Does Water Pressure Increase With Temperature As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. The change in specific volume for a given change in temperature is not the same at various beginning temperatures. So whilst the water temperature remains at less than 100°c, the. Yes, at constant density, the pressure increases as the temperature does:. How Much Does Water Pressure Increase With Temperature.
From rect.blob.core.windows.net
The Surprising Relationship How Temperature Influences Conduction Velocity How Much Does Water Pressure Increase With Temperature The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. So whilst the water temperature remains at less than 100°c, the. As you heat the pressure cooker, the. How Much Does Water Pressure Increase With Temperature.
From ch301.cm.utexas.edu
heating curve How Much Does Water Pressure Increase With Temperature Yes, at constant density, the pressure increases as the temperature does: The change in specific volume for a given change in temperature is not the same at various beginning temperatures. So whilst the water temperature remains at less than 100°c, the. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\).. How Much Does Water Pressure Increase With Temperature.
From www.businessinsider.com
Water thermal expansion rising sea level Business Insider How Much Does Water Pressure Increase With Temperature The change in specific volume for a given change in temperature is not the same at various beginning temperatures. So whilst the water temperature remains at less than 100°c, the. As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. Yes, at constant density, the pressure increases as the temperature does:. How Much Does Water Pressure Increase With Temperature.
From plot.ly
Water Pressure vs. Temperature line chart made by 18youngt plotly How Much Does Water Pressure Increase With Temperature The change in specific volume for a given change in temperature is not the same at various beginning temperatures. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. If you raise. How Much Does Water Pressure Increase With Temperature.
From www.askmattrab.com
Pressure Liquid Pressure, Pascal's Law and Class Ten Science How Much Does Water Pressure Increase With Temperature The change in specific volume for a given change in temperature is not the same at various beginning temperatures. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. So whilst the water temperature remains at less than 100°c, the. Yes, at constant density, the pressure increases. How Much Does Water Pressure Increase With Temperature.
From www.homedit.com
How To Increase Water Pressure In Your Home How Much Does Water Pressure Increase With Temperature The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. So whilst the water temperature remains at less than 100°c, the. The change in specific volume for a given. How Much Does Water Pressure Increase With Temperature.
From blog.thepipingmart.com
Does increasing pipe size increase water pressure? How Much Does Water Pressure Increase With Temperature The change in specific volume for a given change in temperature is not the same at various beginning temperatures. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. Yes, at constant density, the pressure increases as the temperature does: The relationships among the volume of a. How Much Does Water Pressure Increase With Temperature.
From www.researchgate.net
Correlation between temperature and saturation vapor pressure [4 How Much Does Water Pressure Increase With Temperature If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases.. How Much Does Water Pressure Increase With Temperature.
From enduricplumbing.com
How to Increase Water Pressure in Your Home Tips from Pros How Much Does Water Pressure Increase With Temperature So whilst the water temperature remains at less than 100°c, the. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. As you heat the pressure cooker, the water’s. How Much Does Water Pressure Increase With Temperature.
From www.youtube.com
What increase in pressure is required to decrease the volume of 200 How Much Does Water Pressure Increase With Temperature The change in specific volume for a given change in temperature is not the same at various beginning temperatures. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. So whilst the water temperature remains at less than 100°c, the. If you raise the pressure keeping the. How Much Does Water Pressure Increase With Temperature.
From www.engineeringtoolbox.com
Water Saturation Pressure How Much Does Water Pressure Increase With Temperature If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. The change in specific volume for a given change in temperature is not the same at various beginning temperatures.. How Much Does Water Pressure Increase With Temperature.
From exokfsmlv.blob.core.windows.net
How Does Water Pressure Increase With Depth at Don Hill blog How Much Does Water Pressure Increase With Temperature If you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa, or. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). So whilst the water temperature remains at less than 100°c, the. Yes, at constant density, the pressure increases as the temperature does: The. How Much Does Water Pressure Increase With Temperature.
From www.wikihow.com
3 Ways to Increase Water Pressure wikiHow How Much Does Water Pressure Increase With Temperature The pressure increase due to a temperature increase in water can be calculated using the ideal gas law, which states that p1 /. The change in specific volume for a given change in temperature is not the same at various beginning temperatures. As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water. How Much Does Water Pressure Increase With Temperature.