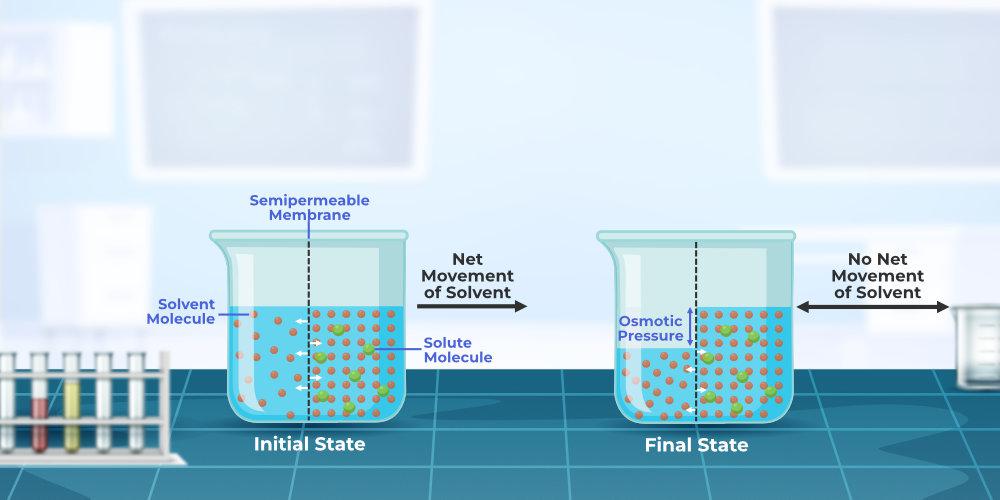
How To Increase Osmotic Pressure . See how to calculate osmotic pressure using the. From a technical standpoint, osmotic pressure can be categorized into two types: Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant and. Potential osmotic pressure and actual osmotic pressure. Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow.
from www.geeksforgeeks.org
Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. From a technical standpoint, osmotic pressure can be categorized into two types: See how to calculate osmotic pressure using the. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant and. Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Potential osmotic pressure and actual osmotic pressure.
Osmosis Definition, Osmotic Pressure, Formula, Examples, & FAQs
How To Increase Osmotic Pressure Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. From a technical standpoint, osmotic pressure can be categorized into two types: Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant and. Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. Potential osmotic pressure and actual osmotic pressure. See how to calculate osmotic pressure using the. Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow.
From www.youtube.com
Osmosis & Osmotic pressure YouTube How To Increase Osmotic Pressure Potential osmotic pressure and actual osmotic pressure. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. See how to calculate osmotic pressure using the. Learn. How To Increase Osmotic Pressure.
From www.slideserve.com
PPT Water Balance PowerPoint Presentation, free download ID4769466 How To Increase Osmotic Pressure See how to calculate osmotic pressure using the. Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Potential osmotic pressure and actual osmotic pressure. Learn how to calculate osmotic pressure using the formula π = icrt,. How To Increase Osmotic Pressure.
From www.pinterest.co.kr
Hydrostatic pressure Vs Oncotic pressure made easy Osmotic pressure How To Increase Osmotic Pressure Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant and. From a technical standpoint, osmotic pressure can be categorized into two types: Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of. How To Increase Osmotic Pressure.
From study.com
Osmotic Pressure Definition & Formula Video & Lesson Transcript How To Increase Osmotic Pressure See how to calculate osmotic pressure using the. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Potential osmotic pressure and actual osmotic pressure. From a technical standpoint, osmotic pressure can be categorized into two types: Osmotic pressure is the pressure that. How To Increase Osmotic Pressure.
From www.biologyonline.com
Osmotic pressure Definition and Examples Biology Online Dictionary How To Increase Osmotic Pressure From a technical standpoint, osmotic pressure can be categorized into two types: Potential osmotic pressure and actual osmotic pressure. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant and. Osmotic pressure is the pressure that would. How To Increase Osmotic Pressure.
From lookfordiagnosis.com
Osmotic Pressure; Osmotic Shock How To Increase Osmotic Pressure Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant. How To Increase Osmotic Pressure.
From www.slideserve.com
PPT Osmosis, Osmotic pressure and Molecular processes PowerPoint How To Increase Osmotic Pressure Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. See how to calculate osmotic pressure using the. Potential osmotic pressure and actual osmotic pressure. From a technical standpoint, osmotic pressure can be categorized into two types: Learn how osmotic pressure is the pressure needed to keep solvent. How To Increase Osmotic Pressure.
From psiberg.com
Osmotic Pressure Determination and Applications How To Increase Osmotic Pressure Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't. How To Increase Osmotic Pressure.
From www.thoughtco.com
Osmotic Pressure and Tonicity How To Increase Osmotic Pressure Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. See how to calculate osmotic pressure using the. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas. How To Increase Osmotic Pressure.
From inspiritvr.com
Osmotic Pressure Study Guide Inspirit How To Increase Osmotic Pressure Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Osmotic pressure is the pressure that would stop water from diffusing. How To Increase Osmotic Pressure.
From www.chemistrylearner.com
Osmotic Pressure Definition, Formula, and Examples How To Increase Osmotic Pressure Potential osmotic pressure and actual osmotic pressure. Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant and. Learn how osmosis. How To Increase Osmotic Pressure.
From www.adda247.com
Osmotic Pressure formula in Biology Class 12 How To Increase Osmotic Pressure Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. From a technical standpoint, osmotic pressure can be categorized into two types: Potential osmotic pressure and actual osmotic pressure. Osmotic pressure is the pressure. How To Increase Osmotic Pressure.
From www.youtube.com
Laws of Osmotic Pressure Solution and Colligative Properties How To Increase Osmotic Pressure Potential osmotic pressure and actual osmotic pressure. See how to calculate osmotic pressure using the. Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Osmotic pressure is the pressure a solvent. How To Increase Osmotic Pressure.
From www.priyamstudycentre.com
Osmosis Definition, Example, Osmotic Pressure How To Increase Osmotic Pressure Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. Potential osmotic pressure and actual osmotic pressure. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't. How To Increase Osmotic Pressure.
From www.geeksforgeeks.org
Osmosis Definition, Osmotic Pressure, Formula, Examples, & FAQs How To Increase Osmotic Pressure Potential osmotic pressure and actual osmotic pressure. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant and. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable. How To Increase Osmotic Pressure.
From www.vrogue.co
Osmotic Pressure Diagram vrogue.co How To Increase Osmotic Pressure Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant and. Potential osmotic pressure and actual osmotic pressure. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable. How To Increase Osmotic Pressure.
From surfguppy.com
Osmotic Pressure Surfguppy Chemistry made easy visual learning How To Increase Osmotic Pressure See how to calculate osmotic pressure using the. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. Learn how osmotic pressure is the pressure needed to. How To Increase Osmotic Pressure.
From www.geeksforgeeks.org
What Is Osmosis? Definition, Types, Osmotic Pressure & Significance How To Increase Osmotic Pressure Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. See how to calculate osmotic pressure using the. Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows. How To Increase Osmotic Pressure.
From www.youtube.com
Osmotic Pressure Osmosis Colligative property Physiology Series How To Increase Osmotic Pressure Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Potential osmotic pressure and actual osmotic pressure. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal. How To Increase Osmotic Pressure.
From thechemistrynotes.com
Osmotic Pressure Definition, Formulas, Laws How To Increase Osmotic Pressure See how to calculate osmotic pressure using the. Potential osmotic pressure and actual osmotic pressure. Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Learn how to calculate osmotic pressure. How To Increase Osmotic Pressure.
From www.youtube.com
Osmotic Pressure Derivation YouTube How To Increase Osmotic Pressure Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant and. Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. Osmotic pressure is the pressure that. How To Increase Osmotic Pressure.
From www.animalia-life.club
Osmotic Pressure How To Increase Osmotic Pressure Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is. How To Increase Osmotic Pressure.
From pubs.acs.org
Osmotic Pressure as Driving Force for Reducing the Size of How To Increase Osmotic Pressure See how to calculate osmotic pressure using the. From a technical standpoint, osmotic pressure can be categorized into two types: Potential osmotic pressure and actual osmotic pressure. Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis.. How To Increase Osmotic Pressure.
From www.youtube.com
Effect of Osmotic Pressure Lab YouTube How To Increase Osmotic Pressure Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a. How To Increase Osmotic Pressure.
From kunduz.com
Osmotic Pressure Definition, Formula, Equation, Significance, Examples How To Increase Osmotic Pressure Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. From a technical standpoint, osmotic pressure can be categorized into two. How To Increase Osmotic Pressure.
From chem.libretexts.org
13.7 Osmotic Pressure Chemistry LibreTexts How To Increase Osmotic Pressure Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. From a technical standpoint, osmotic pressure can be categorized into two types: Potential osmotic pressure and actual osmotic pressure. See how to calculate osmotic pressure using the. Learn how osmotic pressure is the pressure needed to keep solvent. How To Increase Osmotic Pressure.
From preparatorychemistry.com
Osmosis How To Increase Osmotic Pressure Potential osmotic pressure and actual osmotic pressure. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar concentration of the solute, r is the universal gas constant and. Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Osmotic pressure. How To Increase Osmotic Pressure.
From scienceinfo.com
Osmotic Pressure Definition, Formulas, Laws How To Increase Osmotic Pressure Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor, c is the molar. How To Increase Osmotic Pressure.
From slideserve.com
PPT Molarity PowerPoint Presentation ID259016 How To Increase Osmotic Pressure Potential osmotic pressure and actual osmotic pressure. From a technical standpoint, osmotic pressure can be categorized into two types: Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Learn how to calculate osmotic pressure using the formula π = icrt, where i is the van't hoff factor,. How To Increase Osmotic Pressure.
From pharmaceuticalmicrobiologi.blogspot.com
PHARMACEUTICAL MICROBIOLOGY Osmotic Pressure How To Increase Osmotic Pressure Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. See how to calculate osmotic pressure using the. Learn how osmotic pressure is the pressure needed to keep. How To Increase Osmotic Pressure.
From www.coastalwiki.org
Osmosis Coastal Wiki How To Increase Osmotic Pressure Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Learn how osmotic pressure is the pressure needed to keep solvent. How To Increase Osmotic Pressure.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps How To Increase Osmotic Pressure Learn how osmosis is the diffusion of solvent molecules through a semipermeable membrane and how osmotic pressure is the pressure. Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but not solute to flow. Potential osmotic pressure and actual osmotic pressure. Learn how osmotic pressure is the pressure needed to keep solvent. How To Increase Osmotic Pressure.
From chemistrytalk.org
Osmotic Pressure ChemTalk How To Increase Osmotic Pressure Learn how osmotic pressure is the pressure needed to keep solvent from flowing across a semipermeable membrane. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. See how to calculate osmotic pressure using the. Potential osmotic pressure and actual osmotic pressure. Osmotic. How To Increase Osmotic Pressure.
From www.youtube.com
osmosis and osmotic pressure solutions solutions class 12 chemistry How To Increase Osmotic Pressure Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration. Potential osmotic pressure and actual osmotic pressure. See how to calculate osmotic pressure using the. Osmotic pressure is the difference in pressure between two sides of a semipermeable membrane that allows solvent but. How To Increase Osmotic Pressure.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps How To Increase Osmotic Pressure From a technical standpoint, osmotic pressure can be categorized into two types: Osmotic pressure is the pressure that would stop water from diffusing through a barrier by osmosis. See how to calculate osmotic pressure using the. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference. How To Increase Osmotic Pressure.