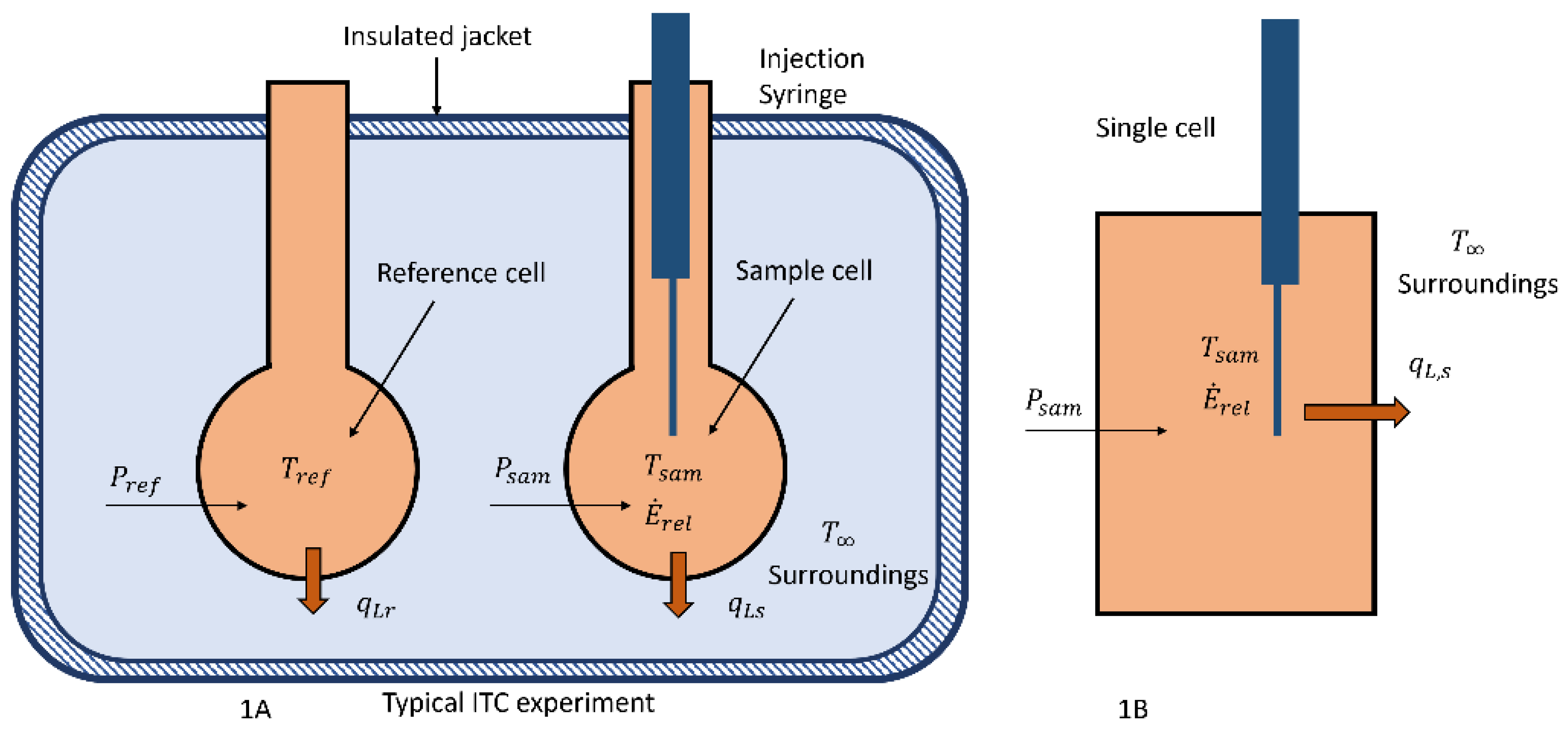
Calorimetric Enthalpy Change . If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Different experiments can be done to measure enthalpy change. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. A calorimeter can be made up of a polystyrene drinking cup, a. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends.
from www.mdpi.com
Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; A calorimeter can be made up of a polystyrene drinking cup, a. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. Different experiments can be done to measure enthalpy change.
Entropy Free FullText Calorimetric Measurements of Biological
Calorimetric Enthalpy Change Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Different experiments can be done to measure enthalpy change. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter can be made up of a polystyrene drinking cup, a.
From evulpo.com
evulpo Standard enthalpy changes Calorimetric Enthalpy Change In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter can be made up of a polystyrene drinking. Calorimetric Enthalpy Change.
From www.slideserve.com
PPT Heat Capacity PowerPoint Presentation, free download ID6674197 Calorimetric Enthalpy Change A calorimeter can be made up of a polystyrene drinking cup, a. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry is a technique used to measure changes in enthalpy of. Calorimetric Enthalpy Change.
From studylib.net
Calorimetry Heat of Neutralization Calorimetric Enthalpy Change The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Different experiments can be done to measure enthalpy change. A. Calorimetric Enthalpy Change.
From www.researchgate.net
Entropies for dryair (N 2 , O 2 , Ar, CO 2 and water H 2 O) species Calorimetric Enthalpy Change The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the. Calorimetric Enthalpy Change.
From www.surfacesciencewestern.com
Differential Scanning Calorimetry (DSC) Surface Science Western Calorimetric Enthalpy Change If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. Calorimetry is a. Calorimetric Enthalpy Change.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:3.1.2 Calorimetry Calorimetric Enthalpy Change Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Different experiments can be done to measure. Calorimetric Enthalpy Change.
From www.learnsci.com
LearnSci LabSim Calorimeter for Measuring Enthalpy Change of Calorimetric Enthalpy Change Different experiments can be done to measure enthalpy change. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. If you know the heat capacity of the system, you can calculate δh using the temperature change. Calorimetric Enthalpy Change.
From www.youtube.com
CHEM 101 Calculating Enthalpy of Solution 2 YouTube Calorimetric Enthalpy Change The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. A calorimeter can be made up of a polystyrene drinking cup, a. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry, as a thermal analysis technique,. Calorimetric Enthalpy Change.
From www.researchgate.net
(a) (i) schematic of an isothermal titration calorimeter and (ii Calorimetric Enthalpy Change Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. If you know the heat capacity of the system, you can. Calorimetric Enthalpy Change.
From haipernews.com
How To Calculate Heat Of Enthalpy Haiper Calorimetric Enthalpy Change The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. Different experiments can be done to measure enthalpy change. Calorimetry,. Calorimetric Enthalpy Change.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetric Enthalpy Change Different experiments can be done to measure enthalpy change. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry is a technique used to measure changes in. Calorimetric Enthalpy Change.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetric Enthalpy Change Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Different experiments can be done to measure enthalpy change. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. A calorimeter can be made up of a polystyrene drinking cup, a. If you know. Calorimetric Enthalpy Change.
From slideplayer.com
Changes in Enthalpy During Chemical Reactions ppt download Calorimetric Enthalpy Change If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In the calorimetric experiment, substances are mixed in an. Calorimetric Enthalpy Change.
From slideplayer.com
Calorimetry. ppt download Calorimetric Enthalpy Change Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. If you know the heat capacity. Calorimetric Enthalpy Change.
From www.researchgate.net
Isothermal titration calorimetric measurements of the binding of Calorimetric Enthalpy Change Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Different experiments can be done to measure enthalpy change. A calorimeter can be made up of a polystyrene drinking cup, a. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. If you know the heat. Calorimetric Enthalpy Change.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetric Enthalpy Change Different experiments can be done to measure enthalpy change. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter can be made up of a polystyrene drinking cup, a. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. If you know. Calorimetric Enthalpy Change.
From www.mdpi.com
Entropy Free FullText Calorimetric Measurements of Biological Calorimetric Enthalpy Change Different experiments can be done to measure enthalpy change. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter can be made up of a polystyrene drinking cup, a. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. If you know the heat capacity of the system, you. Calorimetric Enthalpy Change.
From www.researchgate.net
The plot of temperature vs. enthalpy change for [CnEmpy][Br] Download Calorimetric Enthalpy Change Different experiments can be done to measure enthalpy change. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. A calorimeter can be made up of a polystyrene drinking cup, a. Calorimetry is the set. Calorimetric Enthalpy Change.
From slideplayer.com
A Comprehensive Calorimetric Investigation of an Entropically Driven T Calorimetric Enthalpy Change Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In the calorimetric experiment, substances are mixed in an insulated container,. Calorimetric Enthalpy Change.
From www.youtube.com
CALORIMETRIC METHOD EXPLAINED! (Experimental enthalpy change) YouTube Calorimetric Enthalpy Change In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Calorimetry. Calorimetric Enthalpy Change.
From www.researchgate.net
Calorimetric results for the (Ni 49.5 Mn 50.5x Ti x ) 99.8 B 0.2 Calorimetric Enthalpy Change Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; Different experiments can be done to measure enthalpy change. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only. Calorimetric Enthalpy Change.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记4.1.4 Determining Enthalpy Change of Calorimetric Enthalpy Change Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter can be made up of a polystyrene drinking cup, a. Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. If you know the heat capacity of the system, you can calculate δh. Calorimetric Enthalpy Change.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetric Enthalpy Change The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Different experiments can. Calorimetric Enthalpy Change.
From www.researchgate.net
Isothermal calorimetric titration of tubulin by CIL102 and the plots Calorimetric Enthalpy Change Different experiments can be done to measure enthalpy change. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. If you know. Calorimetric Enthalpy Change.
From users.highland.edu
Calorimetry Calorimetric Enthalpy Change A calorimeter can be made up of a polystyrene drinking cup, a. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. Calorimetry, as a thermal analysis technique, has a broad range of applications. Calorimetric Enthalpy Change.
From www.researchgate.net
(PDF) Calorimetric estimate of the enthalpy change for the substrate Calorimetric Enthalpy Change A calorimeter can be made up of a polystyrene drinking cup, a. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; Calorimetry,. Calorimetric Enthalpy Change.
From www.researchgate.net
(PDF) Calorimetric determination of the enthalpy change for the Calorimetric Enthalpy Change Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. Calorimetry is the. Calorimetric Enthalpy Change.
From slideplayer.com
Unit 5 Energetics. ppt download Calorimetric Enthalpy Change Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. Calorimetry is a technique used to measure changes in enthalpy. Calorimetric Enthalpy Change.
From www.studocu.com
Expt 7 Enthalpy of Combustion (Tr) EXPERIMENT 7 CALORIMETRIC Calorimetric Enthalpy Change If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. A calorimeter can be made up of. Calorimetric Enthalpy Change.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetric Enthalpy Change Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. A calorimeter can be made up of a polystyrene drinking cup, a. Different experiments can be done to measure enthalpy change. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The standard enthalpy. Calorimetric Enthalpy Change.
From www.researchgate.net
Isothermal titration calorimetric experiments of LPS protein mixtures Calorimetric Enthalpy Change Different experiments can be done to measure enthalpy change. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a. Calorimetric Enthalpy Change.
From www.linstitute.net
CIE A Level Chemistry复习笔记5.1.7 Constructing Energy Cycles using Calorimetric Enthalpy Change Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. In the calorimetric experiment, substances are mixed in an insulated container, known as a calorimeter, and. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter can be made up of a polystyrene. Calorimetric Enthalpy Change.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.2 Standard Enthalpy Change翰林国际教育 Calorimetric Enthalpy Change Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Different experiments can be done to measure enthalpy change. Calorimetry, as a thermal analysis technique, has a broad range of applications that include. Calorimetric Enthalpy Change.
From slideplayer.com
Volume 98, Issue 12, Pages (June 2010) ppt download Calorimetric Enthalpy Change If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; In the calorimetric experiment, substances are mixed in an insulated. Calorimetric Enthalpy Change.
From askfilo.com
Enthalpies change in chemical reaction Amount of heat absorbed or evol.. Calorimetric Enthalpy Change Calorimetry is a technique used to measure changes in enthalpy of chemical reactions; If you know the heat capacity of the system, you can calculate δh using the temperature change data from a calorimetry experiment. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of. Calorimetric Enthalpy Change.