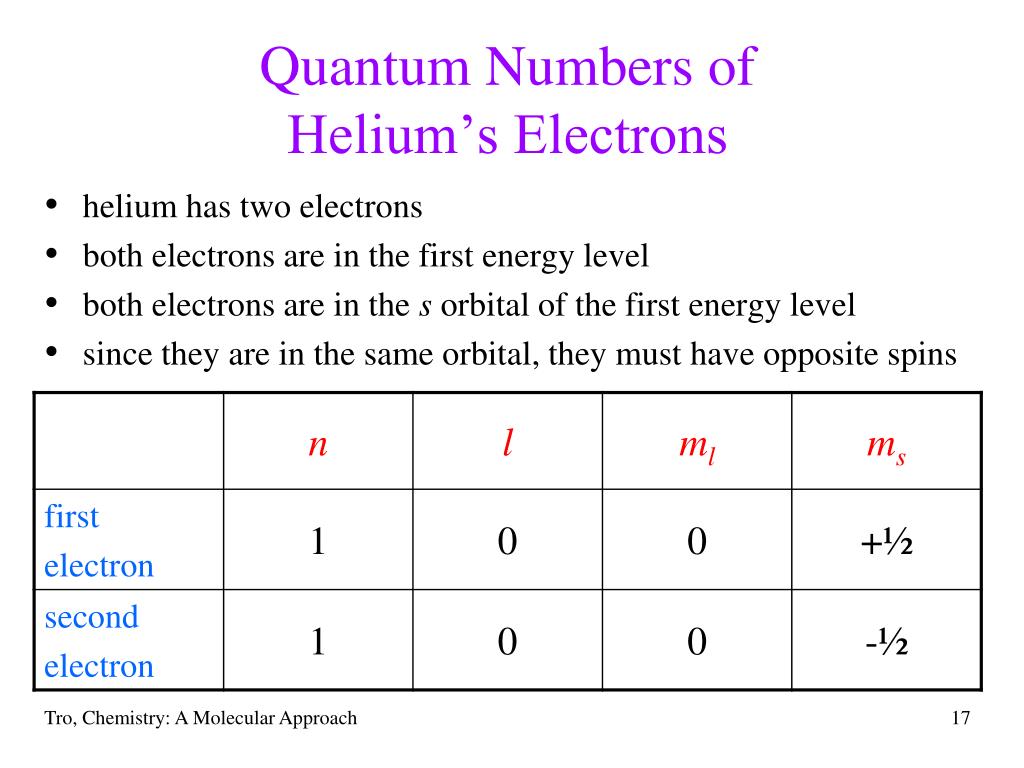
Complete Set Of Quantum Numbers For Helium . Note that, as with hydrogen, the di erence in an electron’s energy. Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. There are four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. N, ℓ, m ℓ, and m s. In atoms, there are a total of four quantum numbers: Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: Each one is a particular factor in an equation describing a property of the electron. 2 are the principal quantum numbers associated with r 1 and r 2, respectively.
from www.slideserve.com
Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. Each one is a particular factor in an equation describing a property of the electron. In atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. N, ℓ, m ℓ, and m s. Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: Note that, as with hydrogen, the di erence in an electron’s energy. There are four quantum numbers: 2 are the principal quantum numbers associated with r 1 and r 2, respectively.
PPT Chapter 8 Periodic Properties of the Elements PowerPoint
Complete Set Of Quantum Numbers For Helium Each one is a particular factor in an equation describing a property of the electron. There are four quantum numbers: Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: Each one is a particular factor in an equation describing a property of the electron. N, ℓ, m ℓ, and m s. Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. Note that, as with hydrogen, the di erence in an electron’s energy. 2 are the principal quantum numbers associated with r 1 and r 2, respectively. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. In atoms, there are a total of four quantum numbers:
From www.researchgate.net
Principal quantum number, types and number of orbitals, maximum number Complete Set Of Quantum Numbers For Helium N, ℓ, m ℓ, and m s. 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Note that, as with hydrogen, the di erence in an electron’s energy. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. Thus the complete set of states can. Complete Set Of Quantum Numbers For Helium.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID5457429 Complete Set Of Quantum Numbers For Helium The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: There are four quantum numbers: Each one is a. Complete Set Of Quantum Numbers For Helium.
From www.slideserve.com
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation Complete Set Of Quantum Numbers For Helium Each one is a particular factor in an equation describing a property of the electron. In atoms, there are a total of four quantum numbers: Note that, as with hydrogen, the di erence in an electron’s energy. N, ℓ, m ℓ, and m s. Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript. Complete Set Of Quantum Numbers For Helium.
From www.slideserve.com
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation Complete Set Of Quantum Numbers For Helium Note that, as with hydrogen, the di erence in an electron’s energy. In atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. There are four quantum numbers: 2 are the principal quantum numbers associated with r 1 and r 2,. Complete Set Of Quantum Numbers For Helium.
From www.youtube.com
Correct set of four quantum numbers for the valence (outermost Complete Set Of Quantum Numbers For Helium The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. Each one is a particular factor in an equation describing a property of the electron. N, ℓ, m ℓ, and m s. Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for. Complete Set Of Quantum Numbers For Helium.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape Complete Set Of Quantum Numbers For Helium N, ℓ, m ℓ, and m s. Each one is a particular factor in an equation describing a property of the electron. Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: There are four quantum numbers: In atoms, there are a total of four quantum numbers: Note that, as with hydrogen, the. Complete Set Of Quantum Numbers For Helium.
From www.slideserve.com
PPT Chapter 8 Periodic Properties of the Elements PowerPoint Complete Set Of Quantum Numbers For Helium Note that, as with hydrogen, the di erence in an electron’s energy. Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: There are four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. The principal quantum number (n), the orbital angular momentum quantum number. Complete Set Of Quantum Numbers For Helium.
From chemistrypubs.com
Quantum Numbers Principle, Examples, Significance Chemistrupubs Complete Set Of Quantum Numbers For Helium In atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. N, ℓ, m ℓ, and m s. There are four quantum numbers: Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for. Complete Set Of Quantum Numbers For Helium.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Complete Set Of Quantum Numbers For Helium Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: 2 are the principal quantum numbers associated with r 1 and r 2, respectively. There are four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. In atoms, there are a total of four quantum. Complete Set Of Quantum Numbers For Helium.
From stock.adobe.com
table of quantum numbers Stock Vector Adobe Stock Complete Set Of Quantum Numbers For Helium 2 are the principal quantum numbers associated with r 1 and r 2, respectively. In atoms, there are a total of four quantum numbers: Note that, as with hydrogen, the di erence in an electron’s energy. There are four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. Spinorbitals can be designated. Complete Set Of Quantum Numbers For Helium.
From shanimchemistry.blogspot.com
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS Complete Set Of Quantum Numbers For Helium Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. Each one is a particular factor in an equation. Complete Set Of Quantum Numbers For Helium.
From brainly.in
Consider the following sets of quantum numbers Brainly.in Complete Set Of Quantum Numbers For Helium Note that, as with hydrogen, the di erence in an electron’s energy. In atoms, there are a total of four quantum numbers: Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. There are four quantum numbers: Each one is a particular factor in an equation describing a property of. Complete Set Of Quantum Numbers For Helium.
From www.pakarkimia.com
quantumnumberchart Ilmu Kimia Complete Set Of Quantum Numbers For Helium Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: N, ℓ, m ℓ, and m s. In atoms, there are a total of four quantum numbers: Note that, as with hydrogen, the di erence in an electron’s energy. Each one is a particular factor in an equation describing a property of the. Complete Set Of Quantum Numbers For Helium.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Complete Set Of Quantum Numbers For Helium Note that, as with hydrogen, the di erence in an electron’s energy. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Each one is a particular factor in an equation describing a property of the. Complete Set Of Quantum Numbers For Helium.
From www.allaboutcircuits.com
Quantum Physics Solidstate Device Theory Electronics Textbook Complete Set Of Quantum Numbers For Helium Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. There are four quantum numbers: Note that, as with hydrogen, the di erence in an electron’s energy. N, ℓ, m ℓ, and m s. Thus the complete set of states can be grouped into two types as distinguished by their. Complete Set Of Quantum Numbers For Helium.
From en.ppt-online.org
Atomic structure and properties. (Chapter 3) online presentation Complete Set Of Quantum Numbers For Helium Each one is a particular factor in an equation describing a property of the electron. Note that, as with hydrogen, the di erence in an electron’s energy. N, ℓ, m ℓ, and m s. Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: In atoms, there are a total of four quantum. Complete Set Of Quantum Numbers For Helium.
From quantum-lesson.blogspot.com
How To Write Set Of Quantum Numbers Complete Set Of Quantum Numbers For Helium Note that, as with hydrogen, the di erence in an electron’s energy. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: Spinorbitals can be designated by a single subscript, for example, \(\phi_a\). Complete Set Of Quantum Numbers For Helium.
From www.chegg.com
Solved A possible set of quantum numbers for the last Complete Set Of Quantum Numbers For Helium In atoms, there are a total of four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. 2 are the principal quantum numbers associated with r 1 and r 2, respectively. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. N,. Complete Set Of Quantum Numbers For Helium.
From www.coursehero.com
[Solved] Complete the table below by providing a set of quantum numbers Complete Set Of Quantum Numbers For Helium 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Each one is a particular factor in an equation describing a property of the electron. In atoms, there are a total of four quantum numbers: Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: N, ℓ, m ℓ,. Complete Set Of Quantum Numbers For Helium.
From www.youtube.com
Quantum Numbers YouTube Complete Set Of Quantum Numbers For Helium In atoms, there are a total of four quantum numbers: There are four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. N, ℓ, m ℓ, and m s. Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for. Complete Set Of Quantum Numbers For Helium.
From www.w3schools.blog
Quantum Numbers W3schools Complete Set Of Quantum Numbers For Helium In atoms, there are a total of four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. N, ℓ, m ℓ, and m s. There are four quantum numbers: Note that, as. Complete Set Of Quantum Numbers For Helium.
From studylib.net
Quantum Numbers Complete Set Of Quantum Numbers For Helium Each one is a particular factor in an equation describing a property of the electron. Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: The principal quantum number (n), the orbital angular. Complete Set Of Quantum Numbers For Helium.
From andreaminsharp.blogspot.com
Set of Quantum Numbers AndreaminSharp Complete Set Of Quantum Numbers For Helium 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: In atoms, there are a total of four quantum. Complete Set Of Quantum Numbers For Helium.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID1160749 Complete Set Of Quantum Numbers For Helium N, ℓ, m ℓ, and m s. Each one is a particular factor in an equation describing a property of the electron. There are four quantum numbers: 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: Note that,. Complete Set Of Quantum Numbers For Helium.
From periodictable.me
How To Find the Helium Electron Configuration (He) Complete Set Of Quantum Numbers For Helium Each one is a particular factor in an equation describing a property of the electron. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. In atoms, there are a total of four quantum numbers: N, ℓ, m ℓ, and m s. Spinorbitals can be designated by a single subscript,. Complete Set Of Quantum Numbers For Helium.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry Complete Set Of Quantum Numbers For Helium Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: N, ℓ, m ℓ, and m s. Note that, as with hydrogen, the di erence in an electron’s energy. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. Each one is a. Complete Set Of Quantum Numbers For Helium.
From www.slideserve.com
PPT Unit 3 Atomic Theory & Quantum Mechanics Sections A.4 A.5 Complete Set Of Quantum Numbers For Helium Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. In atoms, there are a total of four quantum numbers: There are four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. Note that, as with hydrogen,. Complete Set Of Quantum Numbers For Helium.
From www.chegg.com
Solved Complete the table by pairing each set of quantum Complete Set Of Quantum Numbers For Helium N, ℓ, m ℓ, and m s. Note that, as with hydrogen, the di erence in an electron’s energy. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. In atoms, there are a total of four quantum numbers: Thus the complete set of states can be grouped into two. Complete Set Of Quantum Numbers For Helium.
From www.slideserve.com
PPT Orbitals and Quantum Numbers PowerPoint Presentation, free Complete Set Of Quantum Numbers For Helium The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. 2 are the principal quantum numbers associated with r 1 and r 2, respectively. N, ℓ, m ℓ, and m s. There are four quantum numbers: In atoms, there are a total of four quantum numbers: Spinorbitals can be designated. Complete Set Of Quantum Numbers For Helium.
From www.numerade.com
SOLVED Write a complete set of quantum numbers (n, ℓ, and .mℓ) for Complete Set Of Quantum Numbers For Helium There are four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. Thus the complete set of states can be grouped into two types as distinguished by their multiplicities: 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Spinorbitals can be designated by a single subscript, for. Complete Set Of Quantum Numbers For Helium.
From testbook.com
Understand Quantum Numbers Detailed Explanation with Examples Complete Set Of Quantum Numbers For Helium N, ℓ, m ℓ, and m s. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. 2 are the principal quantum numbers associated with r 1 and r 2,. Complete Set Of Quantum Numbers For Helium.
From studylib.net
Quantum Numbers Complete Set Of Quantum Numbers For Helium In atoms, there are a total of four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Thus. Complete Set Of Quantum Numbers For Helium.
From www.slideserve.com
PPT Electron Configuration PowerPoint Presentation, free download Complete Set Of Quantum Numbers For Helium Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. 2 are the principal quantum numbers associated with r 1 and r 2, respectively. In atoms, there are a total of four quantum numbers: Each one is a particular factor in an equation describing a property of the electron. N,. Complete Set Of Quantum Numbers For Helium.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin Complete Set Of Quantum Numbers For Helium The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. N, ℓ, m ℓ, and m s. 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Note that, as with hydrogen, the di erence in an electron’s energy. There are four quantum numbers: In atoms,. Complete Set Of Quantum Numbers For Helium.
From chemistrypubs.com
Quantum Numbers Principle, Examples, Significance Chemistrupubs Complete Set Of Quantum Numbers For Helium Spinorbitals can be designated by a single subscript, for example, \(\phi_a\) or \(\phi_b\), where the subscript stands for a set. N, ℓ, m ℓ, and m s. 2 are the principal quantum numbers associated with r 1 and r 2, respectively. Note that, as with hydrogen, the di erence in an electron’s energy. The principal quantum number (n), the orbital. Complete Set Of Quantum Numbers For Helium.