Methyl Alcohol Soluble In Water . Methanol is soluble in water because it can form hydrogen bonds with water molecules. When the hydrocarbon chain is short, the alcohol is soluble in water. Solubility of alcohols in water. The smaller alcohols (methyl alcohol) are soluble in water because they have. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. To explain the methyl alcohol is soluble in water in all proportion. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Is methanol soluble in water?
from www.chegg.com
Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. To explain the methyl alcohol is soluble in water in all proportion. Is methanol soluble in water? The smaller alcohols (methyl alcohol) are soluble in water because they have. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Solubility of alcohols in water. Methanol is soluble in water because it can form hydrogen bonds with water molecules. When the hydrocarbon chain is short, the alcohol is soluble in water.
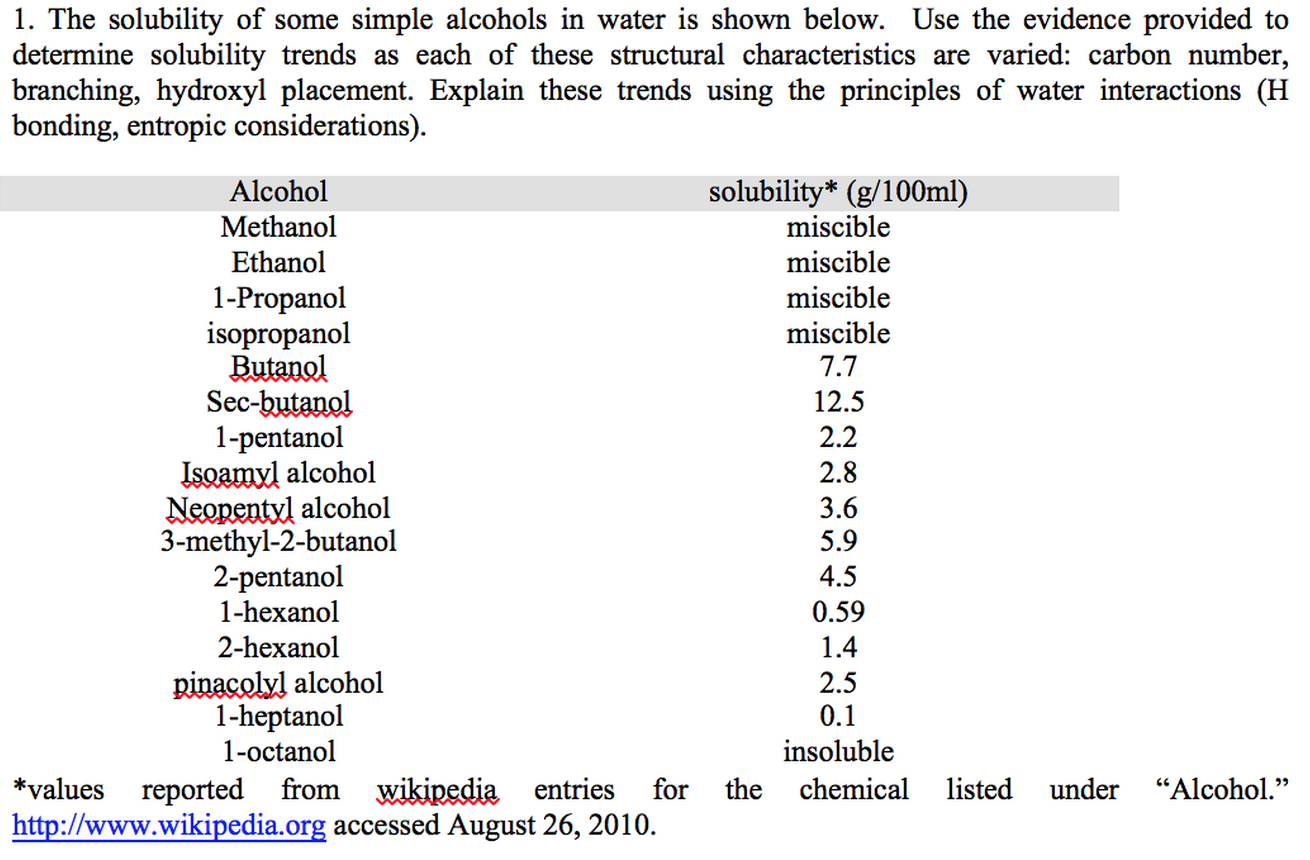
Solved 1. The Solubility Of Some Simple Alcohols In Water...
Methyl Alcohol Soluble In Water To explain the methyl alcohol is soluble in water in all proportion. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. Solubility of alcohols in water. To explain the methyl alcohol is soluble in water in all proportion. When the hydrocarbon chain is short, the alcohol is soluble in water. Is methanol soluble in water? Methanol is soluble in water because it can form hydrogen bonds with water molecules. The smaller alcohols (methyl alcohol) are soluble in water because they have. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2.
From www.researchgate.net
(PDF) Solubility of Fructose in WaterEthanol and WaterMethanol Methyl Alcohol Soluble In Water There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Solubility of alcohols in water. When the hydrocarbon chain is short, the alcohol is soluble in water. Methanol is soluble in water because it can form hydrogen bonds with water molecules. Alcohols and water have the ability to form hydrogen bonds with. Methyl Alcohol Soluble In Water.
From www.semanticscholar.org
Table 2 from Solubility of NaCl, NaBr, and KCl in Water, Methanol Methyl Alcohol Soluble In Water Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. When the hydrocarbon chain is short, the alcohol is soluble in water. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Is methanol soluble in water? Methanol is soluble in water. Methyl Alcohol Soluble In Water.
From www.chegg.com
Solved Consider The Table Shown Here, Which Lists The Sol... Methyl Alcohol Soluble In Water To explain the methyl alcohol is soluble in water in all proportion. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. Is methanol soluble in water? Methanol is soluble in water because it can form hydrogen bonds with water molecules. Solubility of alcohols in water. When the hydrocarbon chain. Methyl Alcohol Soluble In Water.
From www.slideserve.com
PPT Chapter 10 Structure and Synthesis of Alcohols PowerPoint Methyl Alcohol Soluble In Water To explain the methyl alcohol is soluble in water in all proportion. Methanol is soluble in water because it can form hydrogen bonds with water molecules. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. There is no limit on the amount of methanol (ch 3 oh) and ethanol. Methyl Alcohol Soluble In Water.
From www.semanticscholar.org
Figure 5 from Solubility of NaCl, NaBr, and KCl in Water, Methanol Methyl Alcohol Soluble In Water To explain the methyl alcohol is soluble in water in all proportion. The smaller alcohols (methyl alcohol) are soluble in water because they have. Solubility of alcohols in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. Methanol is soluble in water because it can form hydrogen bonds. Methyl Alcohol Soluble In Water.
From saylordotorg.github.io
Physical Properties of Alcohols Methyl Alcohol Soluble In Water Solubility of alcohols in water. To explain the methyl alcohol is soluble in water in all proportion. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The smaller alcohols (methyl alcohol) are soluble in water because they have. When the hydrocarbon chain is short, the alcohol is soluble in. Methyl Alcohol Soluble In Water.
From www.numerade.com
Experiment 2 Exercise Panel Ed Data Table Panel Data Table Solubility Methyl Alcohol Soluble In Water Methanol is soluble in water because it can form hydrogen bonds with water molecules. To explain the methyl alcohol is soluble in water in all proportion. Is methanol soluble in water? When the hydrocarbon chain is short, the alcohol is soluble in water. Solubility of alcohols in water. The smaller alcohols (methyl alcohol) are soluble in water because they have.. Methyl Alcohol Soluble In Water.
From www.numerade.com
Explain why methyl alcohol (methanol) is soluble Methyl Alcohol Soluble In Water Methanol is soluble in water because it can form hydrogen bonds with water molecules. Solubility of alcohols in water. When the hydrocarbon chain is short, the alcohol is soluble in water. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Alcohols and water have the ability to form hydrogen bonds with. Methyl Alcohol Soluble In Water.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID6009487 Methyl Alcohol Soluble In Water When the hydrocarbon chain is short, the alcohol is soluble in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. Solubility of alcohols in water. To explain the methyl alcohol is soluble in water in all proportion. Is methanol soluble in water? Methanol is soluble in water because. Methyl Alcohol Soluble In Water.
From www.numerade.com
SOLVED Solvents Hexane Water Alcohols Methyl alcohol CH3OH 1Butanol Methyl Alcohol Soluble In Water To explain the methyl alcohol is soluble in water in all proportion. Solubility of alcohols in water. Methanol is soluble in water because it can form hydrogen bonds with water molecules. When the hydrocarbon chain is short, the alcohol is soluble in water. The smaller alcohols (methyl alcohol) are soluble in water because they have. Alcohols and water have the. Methyl Alcohol Soluble In Water.
From www.researchgate.net
Solubility curve of IDA in methanol, ethanol, acetonitrile, acetone and Methyl Alcohol Soluble In Water There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. When the hydrocarbon chain is short, the alcohol is soluble in water. To explain the methyl alcohol is soluble in water in all proportion. Is methanol soluble in water? Alcohols and water have the ability to form hydrogen bonds with one another. Methyl Alcohol Soluble In Water.
From www.researchgate.net
Solubility comparison in methanol. Download Scientific Diagram Methyl Alcohol Soluble In Water When the hydrocarbon chain is short, the alcohol is soluble in water. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. Solubility of alcohols in water. To explain the methyl alcohol. Methyl Alcohol Soluble In Water.
From rayb78.github.io
Solubility In Water Chart Methyl Alcohol Soluble In Water There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Solubility of alcohols in water. To explain the methyl alcohol is soluble in water in all proportion. Methanol is soluble in water because it can form hydrogen bonds with water molecules. The smaller alcohols (methyl alcohol) are soluble in water because they. Methyl Alcohol Soluble In Water.
From mungfali.com
Solubility Rules Flowchart Chart Chemistry Methyl Alcohol Soluble In Water Methanol is soluble in water because it can form hydrogen bonds with water molecules. Is methanol soluble in water? The smaller alcohols (methyl alcohol) are soluble in water because they have. When the hydrocarbon chain is short, the alcohol is soluble in water. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch. Methyl Alcohol Soluble In Water.
From www.youtube.com
4 2 4 alcohols solubility and solvent polarity YouTube Methyl Alcohol Soluble In Water Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. The smaller alcohols (methyl alcohol) are soluble in water because they have. Methanol is soluble in water because it can form hydrogen. Methyl Alcohol Soluble In Water.
From www.researchgate.net
Solubility of CH 4 in water and alcohols Download Table Methyl Alcohol Soluble In Water There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Solubility of alcohols in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. The smaller alcohols (methyl alcohol) are soluble in water because they have. Is methanol soluble in water?. Methyl Alcohol Soluble In Water.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Methyl Alcohol Soluble In Water Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. To explain the methyl alcohol is soluble in water in all proportion. Is methanol soluble in water? There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Methanol is soluble in water. Methyl Alcohol Soluble In Water.
From slideplayer.com
melting & boiling points ppt download Methyl Alcohol Soluble In Water The smaller alcohols (methyl alcohol) are soluble in water because they have. Methanol is soluble in water because it can form hydrogen bonds with water molecules. Is methanol soluble in water? There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Alcohols and water have the ability to form hydrogen bonds with. Methyl Alcohol Soluble In Water.
From www.chegg.com
Solved ALCOHOLS, PHENOLS & CARBOXYLIC ACIDS 1. Solubility in Methyl Alcohol Soluble In Water When the hydrocarbon chain is short, the alcohol is soluble in water. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Solubility of alcohols in water. The smaller alcohols (methyl alcohol) are soluble in water because they have. Alcohols and water have the ability to form hydrogen bonds with one another. Methyl Alcohol Soluble In Water.
From www.researchgate.net
Vapor pressure of methanol and ethanol as a function of temperature Methyl Alcohol Soluble In Water To explain the methyl alcohol is soluble in water in all proportion. Methanol is soluble in water because it can form hydrogen bonds with water molecules. Solubility of alcohols in water. The smaller alcohols (methyl alcohol) are soluble in water because they have. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch. Methyl Alcohol Soluble In Water.
From www.researchgate.net
Benzoic acid solubility in pure water and aqueous methanol fractions Methyl Alcohol Soluble In Water Methanol is soluble in water because it can form hydrogen bonds with water molecules. The smaller alcohols (methyl alcohol) are soluble in water because they have. Is methanol soluble in water? Solubility of alcohols in water. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. There is no limit. Methyl Alcohol Soluble In Water.
From www.researchgate.net
Solubility curve of USA in mixed solvents (water + ethanol) and Methyl Alcohol Soluble In Water Methanol is soluble in water because it can form hydrogen bonds with water molecules. Solubility of alcohols in water. When the hydrocarbon chain is short, the alcohol is soluble in water. Is methanol soluble in water? Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. There is no limit. Methyl Alcohol Soluble In Water.
From slideplayer.com
Alcohols. ppt download Methyl Alcohol Soluble In Water There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. The smaller alcohols (methyl alcohol) are soluble in water because they have. When the hydrocarbon chain is short, the alcohol is soluble in water. Is methanol soluble in water? Methanol is soluble in water because it can form hydrogen bonds with water. Methyl Alcohol Soluble In Water.
From www.numerade.com
SOLVED Text Team Structures of Alcohols and Phenols Fill in the Methyl Alcohol Soluble In Water There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Methanol is soluble in water because it can form hydrogen bonds with water molecules. The smaller alcohols (methyl alcohol) are soluble in water because they have. Is methanol soluble in water? Solubility of alcohols in water. Alcohols and water have the ability. Methyl Alcohol Soluble In Water.
From www.chegg.com
Solved 2. Consider the following solubility data (in table Methyl Alcohol Soluble In Water There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. When the hydrocarbon chain is short, the alcohol is soluble in water. To explain the methyl alcohol is soluble in water in all proportion. Solubility of alcohols in water. Is methanol soluble in water? Methanol is soluble in water because it can. Methyl Alcohol Soluble In Water.
From www.chegg.com
Solved 1. The Solubility Of Some Simple Alcohols In Water... Methyl Alcohol Soluble In Water When the hydrocarbon chain is short, the alcohol is soluble in water. Solubility of alcohols in water. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. To explain the methyl alcohol is soluble in water in all proportion. Is methanol soluble in water? Methanol is soluble in water because it can. Methyl Alcohol Soluble In Water.
From slideplayer.com
Solutions L. Breen Chemistry ppt download Methyl Alcohol Soluble In Water To explain the methyl alcohol is soluble in water in all proportion. Is methanol soluble in water? There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. Methanol is soluble in water. Methyl Alcohol Soluble In Water.
From www.nagwa.com
Question Video Identifying the Alcohol That Is the Least Soluble in Methyl Alcohol Soluble In Water The smaller alcohols (methyl alcohol) are soluble in water because they have. Methanol is soluble in water because it can form hydrogen bonds with water molecules. To explain the methyl alcohol is soluble in water in all proportion. When the hydrocarbon chain is short, the alcohol is soluble in water. There is no limit on the amount of methanol (ch. Methyl Alcohol Soluble In Water.
From ecclab.empowershop.co.jp
楽天市場・ラクマ、五輪を一年後に控え、スポーツ関連の商品の需要が急上昇 EC業界ニュース・まとめ・コラム「eコマースコンバージョンラボ」 Methyl Alcohol Soluble In Water Solubility of alcohols in water. To explain the methyl alcohol is soluble in water in all proportion. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. When the hydrocarbon chain is short, the alcohol is soluble in water. Is methanol soluble in water? Methanol is soluble in water because it can. Methyl Alcohol Soluble In Water.
From www.researchgate.net
. Methanol and ethanol are sufficiently miscible with water that Methyl Alcohol Soluble In Water Is methanol soluble in water? When the hydrocarbon chain is short, the alcohol is soluble in water. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Solubility of alcohols in water. To explain the methyl alcohol is soluble in water in all proportion. The smaller alcohols (methyl alcohol) are soluble in. Methyl Alcohol Soluble In Water.
From www.researchgate.net
Methanol, ethanol and water adsorption isotherms for 2. Saturation Methyl Alcohol Soluble In Water Is methanol soluble in water? When the hydrocarbon chain is short, the alcohol is soluble in water. Solubility of alcohols in water. The smaller alcohols (methyl alcohol) are soluble in water because they have. To explain the methyl alcohol is soluble in water in all proportion. Methanol is soluble in water because it can form hydrogen bonds with water molecules.. Methyl Alcohol Soluble In Water.
From www.numerade.com
SOLVED Text Physical Properties of Alcohols and Phenols Alcohol pH Methyl Alcohol Soluble In Water Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Methanol is soluble in water because it can form hydrogen bonds with water molecules. Solubility of alcohols in water. When the hydrocarbon. Methyl Alcohol Soluble In Water.
From rayb78.github.io
Solubility In Water Chart Methyl Alcohol Soluble In Water Alcohols and water have the ability to form hydrogen bonds with one another which tends to make the two liquids. There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Methanol is soluble in water because it can form hydrogen bonds with water molecules. Solubility of alcohols in water. To explain the. Methyl Alcohol Soluble In Water.
From www.numerade.com
SOLVEDExplain why methyl alcohol is soluble in water in all Methyl Alcohol Soluble In Water There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. Is methanol soluble in water? Solubility of alcohols in water. To explain the methyl alcohol is soluble in water in all proportion. The smaller alcohols (methyl alcohol) are soluble in water because they have. Alcohols and water have the ability to form. Methyl Alcohol Soluble In Water.
From www.semanticscholar.org
Table 1 from Solubility of NaCl, NaBr, and KCl in Water, Methanol Methyl Alcohol Soluble In Water Solubility of alcohols in water. The smaller alcohols (methyl alcohol) are soluble in water because they have. Methanol is soluble in water because it can form hydrogen bonds with water molecules. Is methanol soluble in water? There is no limit on the amount of methanol (ch 3 oh) and ethanol (ch 3 ch 2. When the hydrocarbon chain is short,. Methyl Alcohol Soluble In Water.