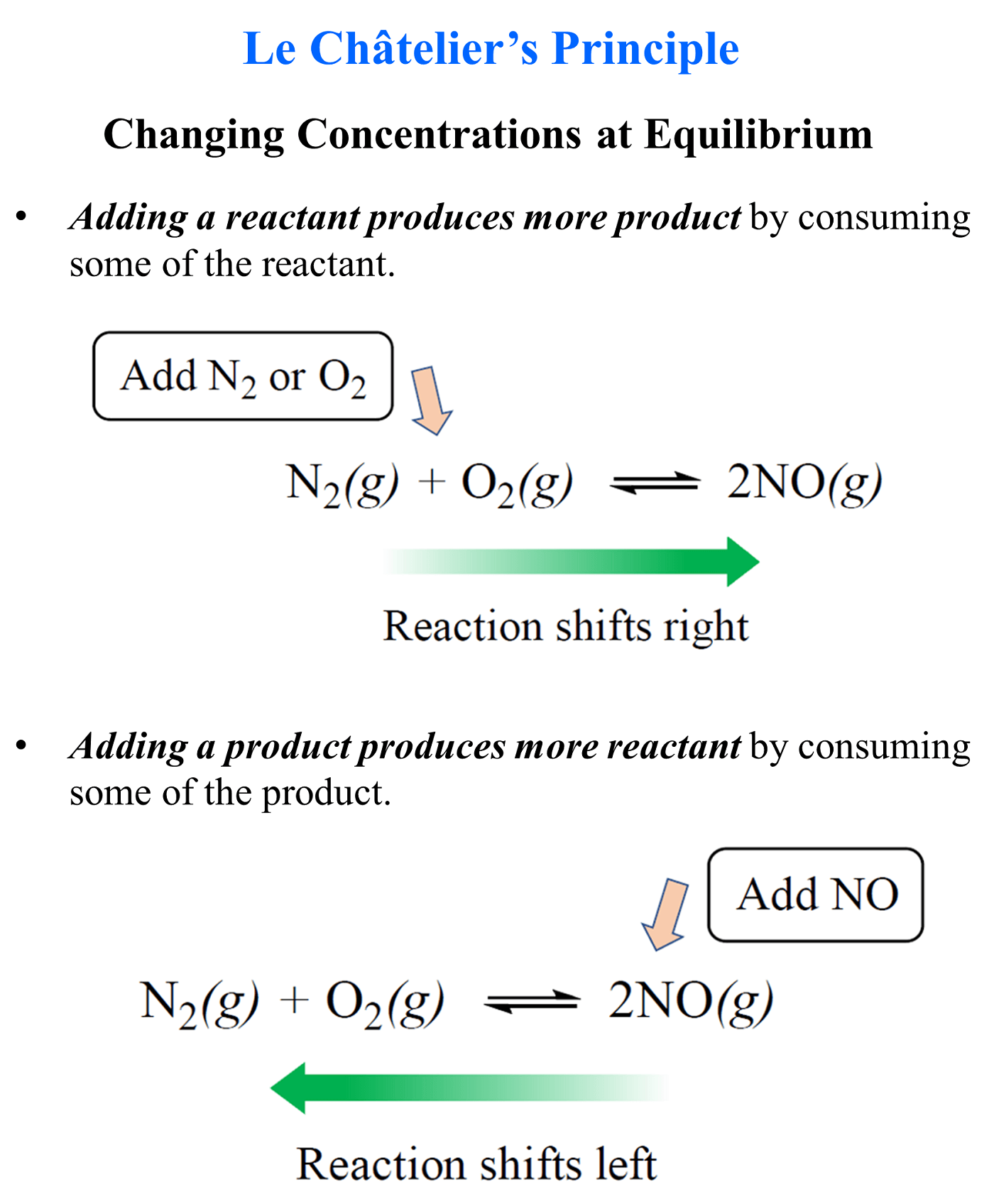Le Chatelier's Principle Solids And Liquids . A system at equilibrium is in a state of dynamic balance,. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. Le chatelier's principle with pure solids and liquids. Predict the response of a stressed equilibrium using le châtelier’s principle. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a.
from general.chemistrysteps.com
A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Le chatelier's principle with pure solids and liquids. A system at equilibrium is in a state of dynamic balance,. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Predict the response of a stressed equilibrium using le châtelier’s principle.
Le Chateliers Principle Chemistry Steps
Le Chatelier's Principle Solids And Liquids Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. Le chatelier's principle with pure solids and liquids. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. Predict the response of a stressed equilibrium using le châtelier’s principle. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. A system at equilibrium is in a state of dynamic balance,. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Le Chatelier's Principle Solids And Liquids Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Le chatelier's principle with pure solids and liquids. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. Learn about le chatelier's principle. Le Chatelier's Principle Solids And Liquids.
From slideplayer.com
Equilibrium and Le Chatelier Principle ppt download Le Chatelier's Principle Solids And Liquids Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Le chatelier's principle with pure solids and liquids. A system at equilibrium is in a state of dynamic balance,. Le chatelier's principle. Le Chatelier's Principle Solids And Liquids.
From www.pinterest.com
Le Chatelier’s Principle Concentration and Pressure Change College Le Chatelier's Principle Solids And Liquids Le chatelier's principle with pure solids and liquids. Predict the response of a stressed equilibrium using le châtelier’s principle. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. A(s) ↽−−⇀ b(g) +c(g) a. Le Chatelier's Principle Solids And Liquids.
From www.youtube.com
Le Chatelier's Principle Animation YouTube Le Chatelier's Principle Solids And Liquids A system at equilibrium is in a state of dynamic balance,. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Le chatelier's principle with pure solids and liquids. A(s) ↽−−⇀ b(g) +c(g) a. Le Chatelier's Principle Solids And Liquids.
From www.pinterest.es
Le Chatelier's Principle explained. Chemistry lessons, Chemistry help Le Chatelier's Principle Solids And Liquids A system at equilibrium is in a state of dynamic balance,. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Le chatelier's principle with pure solids and liquids. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Learn about le. Le Chatelier's Principle Solids And Liquids.
From socratic.org
What is Le Chatelier's principle in chemistry? Socratic Le Chatelier's Principle Solids And Liquids Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Le chatelier's. Le Chatelier's Principle Solids And Liquids.
From demonstrations.wolfram.com
Le Chatelier's Principle in Chemical Equilibrium Wolfram Le Chatelier's Principle Solids And Liquids A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. A system at equilibrium. Le Chatelier's Principle Solids And Liquids.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Le Chatelier's Principle Solids And Liquids A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. Le Chatelier's Principle Solids And Liquids.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Le Chatelier's Principle Solids And Liquids Le chatelier's principle with pure solids and liquids. Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. A system at equilibrium is in a state of dynamic balance,. Predict the response. Le Chatelier's Principle Solids And Liquids.
From chemistryteory.blogspot.com
chemistry Le Chatelier’s Principle Concentration and Pressure Change Le Chatelier's Principle Solids And Liquids Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. A system at equilibrium is in a state of dynamic balance,. A(s) ↽−−⇀. Le Chatelier's Principle Solids And Liquids.
From www.toppr.com
How do you explain Le Chatelier's principle? Le Chatelier's Principle Solids And Liquids Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. Le Chatelier's Principle Solids And Liquids.
From www.youtube.com
According to leChatelier's principle, adding heat to a solid and Le Chatelier's Principle Solids And Liquids Le chatelier's principle with pure solids and liquids. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. A system at equilibrium is. Le Chatelier's Principle Solids And Liquids.
From schoolbag.info
LE CHÂTELIER’S PRINCIPLE CHEMICAL EQUILIBRIUM CHEMISTRY THE CENTRAL Le Chatelier's Principle Solids And Liquids Predict the response of a stressed equilibrium using le châtelier’s principle. A system at equilibrium is in a state of dynamic balance,. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b. Le Chatelier's Principle Solids And Liquids.
From askfilo.com
According to LeChatelier's principle adding heat to a solid and liquid i.. Le Chatelier's Principle Solids And Liquids A system at equilibrium is in a state of dynamic balance,. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Predict the response of a stressed equilibrium using le châtelier’s principle. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts.. Le Chatelier's Principle Solids And Liquids.
From byjus.com
Explain Henry's Law through Le chatelier principles. And also derive Le Chatelier's Principle Solids And Liquids Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Le chatelier's principle with pure solids and liquids. When a chemical system at. Le Chatelier's Principle Solids And Liquids.
From www.youtube.com
According to Le Chatelier's principle, adding heat to a solid and Le Chatelier's Principle Solids And Liquids Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. A system at equilibrium is in a state of dynamic balance,. Le chatelier's principle and how to use it to. Le Chatelier's Principle Solids And Liquids.
From chemistnotes.com
State Le chatelier's principle with applications Chemistry Notes Le Chatelier's Principle Solids And Liquids Predict the response of a stressed equilibrium using le châtelier’s principle. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Learn about le chatelier's principle in chemistry and see examples showing how to predict the. Le Chatelier's Principle Solids And Liquids.
From askfilo.com
According to Lechatelier's principle, adding heat to a solid ⇌ liquid eq.. Le Chatelier's Principle Solids And Liquids Predict the response of a stressed equilibrium using le châtelier’s principle. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. Learn about le chatelier's. Le Chatelier's Principle Solids And Liquids.
From www.brainkart.com
LeChatelier's Principle Le Chatelier's Principle Solids And Liquids Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Le chatelier's principle with pure solids and liquids. Learn about le chatelier's principle in chemistry and see. Le Chatelier's Principle Solids And Liquids.
From readchemistry.com
Le Chatelier’s principle Read Chemistry Le Chatelier's Principle Solids And Liquids A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Learn about le chatelier's principle in. Le Chatelier's Principle Solids And Liquids.
From www.youtube.com
Le Chatelier’s Principle Explained with Examples YouTube Le Chatelier's Principle Solids And Liquids When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. Predict the response of a stressed equilibrium using le châtelier’s principle. Le chatelier's principle with pure solids and liquids. A(s) ↽−−⇀ b(g) +c(g) a. Le Chatelier's Principle Solids And Liquids.
From www.tes.com
Le Chatelier's Principle Teaching Resources Le Chatelier's Principle Solids And Liquids When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. A system at equilibrium is in a state of dynamic balance,. Le chatelier's principle with pure solids and liquids. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a. Le Chatelier's Principle Solids And Liquids.
From www.sliderbase.com
Equilibrium and Le Chatelier's Principle Presentation Chemistry Le Chatelier's Principle Solids And Liquids Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction.. Le Chatelier's Principle Solids And Liquids.
From in.pinterest.com
Le chatelier Priciple Le chatelier's principle, Chemistry, Principles Le Chatelier's Principle Solids And Liquids Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. A system at equilibrium is in a state of dynamic balance,. Predict the response of a stressed equilibrium using le châtelier’s principle. Le chatelier's principle with pure solids and liquids. Learn about le. Le Chatelier's Principle Solids And Liquids.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Le Chatelier's Principle Solids And Liquids Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. Predict the response of a stressed equilibrium using le châtelier’s principle. A system at equilibrium is in a state of dynamic balance,. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance.. Le Chatelier's Principle Solids And Liquids.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Le Chatelier's Principle Solids And Liquids A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. Predict the response of a stressed equilibrium using le châtelier’s principle. Le chatelier's principle states. Le Chatelier's Principle Solids And Liquids.
From slideplayer.com
& Equilibrium ppt download Le Chatelier's Principle Solids And Liquids When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position. Le Chatelier's Principle Solids And Liquids.
From www.researchgate.net
The implementation of the Le Chatelier principle for the evaporation of Le Chatelier's Principle Solids And Liquids Le chatelier's principle with pure solids and liquids. Predict the response of a stressed equilibrium using le châtelier’s principle. Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium. Le Chatelier's Principle Solids And Liquids.
From www.numerade.com
SOLVED LE CHATELIER'S PRINCIPLE Le Chatelier's Principle states that Le Chatelier's Principle Solids And Liquids Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. A system at equilibrium is in a state of dynamic balance,.. Le Chatelier's Principle Solids And Liquids.
From quizlet.com
Le Chatelier's Principle and Equilibrium Diagram Quizlet Le Chatelier's Principle Solids And Liquids Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. When a chemical system at equilibrium is disturbed, it returns to. Le Chatelier's Principle Solids And Liquids.
From www.slideserve.com
PPT Le Châtelier’s Principle PowerPoint Presentation, free download Le Chatelier's Principle Solids And Liquids A system at equilibrium is in a state of dynamic balance,. Predict the response of a stressed equilibrium using le châtelier’s principle. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Le chatelier's principle and. Le Chatelier's Principle Solids And Liquids.
From pdfprof.com
le chatelier principle pdf Le Chatelier's Principle Solids And Liquids Le chatelier's principle with pure solids and liquids. A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Le chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts. A system at equilibrium is in a state of dynamic balance,. Learn about le. Le Chatelier's Principle Solids And Liquids.
From morioh.com
Le Chatelier's Principle Le Chatelier's Principle Solids And Liquids Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. Le chatelier's principle with pure solids and liquids. Predict the response of a stressed equilibrium using le châtelier’s principle. A system at. Le Chatelier's Principle Solids And Liquids.
From www.vedantu.com
Le Chatelier’s Principle Laws, Principle Example and FAQs for JEE Le Chatelier's Principle Solids And Liquids When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. A system at equilibrium is in a state of dynamic balance,. Learn about le chatelier's principle in chemistry and see examples showing how to predict the shift in equilibrium of a chemical reaction. Le chatelier's principle with pure solids and liquids. A(s) ↽−−⇀ b(g). Le Chatelier's Principle Solids And Liquids.
From sciencenotes.org
Le Chatelier's Principle Le Chatelier's Principle Solids And Liquids A(s) ↽−−⇀ b(g) +c(g) a (s) ↽ − − ⇀ b (g) + c (g) since adding a. Predict the response of a stressed equilibrium using le châtelier’s principle. Le chatelier's principle and how to use it to work out what happens to the position of equilibrium if the conditions are changed for a reaction which. Le chatelier's principle with. Le Chatelier's Principle Solids And Liquids.