Medical Device List Eu . The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Welcome to the eudamed information centre. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years. Functional specifications for the european database on medical devices (eudamed) The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. The central point of support for eudamed users, presenting action steps and process logic from.
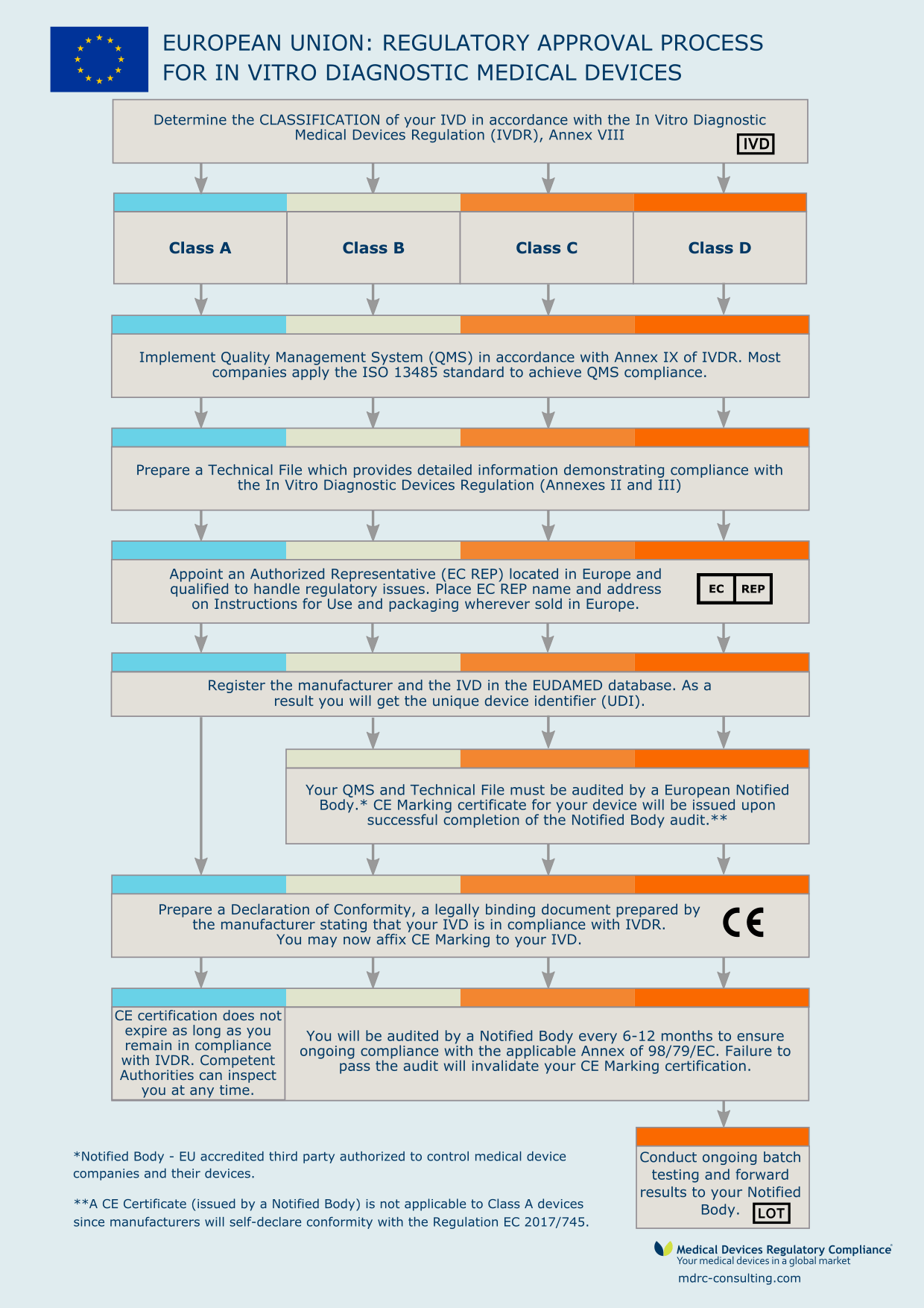
from mdrc-consulting.com
In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. Functional specifications for the european database on medical devices (eudamed) Welcome to the eudamed information centre. The central point of support for eudamed users, presenting action steps and process logic from. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Medical devices are products or equipment intended for a medical purpose. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years.
Downloads MDRC
Medical Device List Eu The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. Medical devices are products or equipment intended for a medical purpose. Welcome to the eudamed information centre. Functional specifications for the european database on medical devices (eudamed) The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a conformity. The central point of support for eudamed users, presenting action steps and process logic from. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years.
From pepgra.com
Medical Device Classification In The European Union pepgra Medical Device List Eu The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. The central point of support for eudamed users, presenting action steps and process logic from. The eu revised the laws governing medical devices and in vitro diagnostics to align with. Medical Device List Eu.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device List Eu In the european union (eu) they must undergo a conformity. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. The central point of support for eudamed users, presenting action steps and process logic from. Functional specifications for the european database on medical devices (eudamed) Regulation (eu) 2017/745 of the european parliament and of. Medical Device List Eu.
From www.auxergo.com
The Interactive Guide Under The New EU Regulations on Medical Devices Medical Device List Eu The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years. Functional specifications for the european database on medical devices (eudamed) The european medical device nomenclature (emdn) is the nomenclature of use. Medical Device List Eu.
From mdrc-consulting.com
Downloads MDRC Medical Device List Eu Welcome to the eudamed information centre. Medical devices are products or equipment intended for a medical purpose. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices,. Medical Device List Eu.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Medical Device List Eu Medical devices are products or equipment intended for a medical purpose. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. The central point of support for eudamed users, presenting action steps and process logic from. Functional specifications for the european database on medical devices (eudamed) Regulation (eu) 2017/745 of the european parliament and. Medical Device List Eu.
From www.scribd.com
European_MDR_Readiness_Checklist_Fillable Quality Management System Medical Device List Eu The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Functional specifications for the european database on medical devices (eudamed) Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.. Medical Device List Eu.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device List Eu The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years. Welcome to the eudamed information centre. Functional specifications for the european database on medical devices (eudamed) Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. Medical Device List Eu.
From www.researchgate.net
List of Current European Union Medical Device Regulations and Medical Device List Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. Welcome to the eudamed information centre. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. In the european union (eu) they must undergo a. Medical Device List Eu.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Medical Device List Eu In the european union (eu) they must undergo a conformity. Functional specifications for the european database on medical devices (eudamed) Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. The central point of support for eudamed users, presenting. Medical Device List Eu.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 Medical Device List Eu The central point of support for eudamed users, presenting action steps and process logic from. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Welcome to the eudamed information centre. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. Medical devices are products or. Medical Device List Eu.
From clin-r.com
EU MDR how to structure your Medical Device Technical Document Clin R Medical Device List Eu The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Functional specifications for the european database on medical devices (eudamed) Medical devices are products or equipment intended for a medical purpose. The central point. Medical Device List Eu.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device List Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Welcome to the eudamed information centre. The central point of support for eudamed users, presenting action steps and process logic from. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Functional specifications for. Medical Device List Eu.
From meaningkosh.com
Mdr Medical Device Classifications MeaningKosh Medical Device List Eu Functional specifications for the european database on medical devices (eudamed) Welcome to the eudamed information centre. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. The european commission published implementing decision (eu) 2019/939,. Medical Device List Eu.
From www.ce-marking.com
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Medical Device List Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Functional specifications for the european database on medical devices (eudamed) The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. In the european union (eu) they must undergo a conformity. Welcome to the eudamed information centre.. Medical Device List Eu.
From emmainternational.com
Classifying Medical Devices under EU MDR Medical Device List Eu The central point of support for eudamed users, presenting action steps and process logic from. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. Functional specifications for the european database on medical devices (eudamed) Welcome to the eudamed information centre. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering. Medical Device List Eu.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device List Eu The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Medical devices are products or equipment intended for a medical purpose. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years. Welcome to the eudamed information centre. Regulation (eu). Medical Device List Eu.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Medical Device List Eu Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Welcome to the eudamed information centre. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. The eu revised. Medical Device List Eu.
From www.youtube.com
European Medical Device Registration Chapter 1 Overview YouTube Medical Device List Eu The central point of support for eudamed users, presenting action steps and process logic from. Welcome to the eudamed information centre. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. Functional specifications for the european database on medical devices (eudamed) Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017. Medical Device List Eu.
From www.scribd.com
List of medical devices Implant (Medicine) Urinary Incontinence Medical Device List Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Functional specifications for the european database on medical devices (eudamed) In the european union (eu) they must undergo a conformity. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. The central point of. Medical Device List Eu.
From learn.marsdd.com
Medical device submissions Placing a medical device on the market Medical Device List Eu Functional specifications for the european database on medical devices (eudamed) The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years. Welcome to the eudamed information centre. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. Medical Device List Eu.
From omcmedical.com
EU Classification of Medical Devices OMC Medical Medical Device List Eu The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Functional specifications for the european. Medical Device List Eu.
From www.i3cglobal.com
EU Medical Device Classification Examples and Rules Medical Device List Eu The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Welcome to the eudamed information centre. Functional specifications for the european database on medical devices (eudamed) The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years. Medical devices are. Medical Device List Eu.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device List Eu The central point of support for eudamed users, presenting action steps and process logic from. In the european union (eu) they must undergo a conformity. Welcome to the eudamed information centre. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. The european commission published implementing decision (eu) 2019/939, designating four entities to assign. Medical Device List Eu.
From medicaldevicehq.com
Different classifications rules for medical device software An Medical Device List Eu The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years. Functional specifications for the european database on medical devices (eudamed) The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Medical devices are products or equipment intended for a. Medical Device List Eu.
From operonstrategist.com
Medical Device Classification EU MDR Guide) Operon Strategist Medical Device List Eu Medical devices are products or equipment intended for a medical purpose. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Welcome to the eudamed information centre. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. The eu revised the laws governing medical devices and in vitro diagnostics to. Medical Device List Eu.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Medical Device List Eu The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. The eu revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector over the last 20 years. In the european union (eu) they must undergo a conformity. Welcome to the eudamed information centre. The central point of support. Medical Device List Eu.
From www.auth-rep.com
Guide on medical devices (MD/IVD) CE marking (mark) & European (EEA/EU Medical Device List Eu The central point of support for eudamed users, presenting action steps and process logic from. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. Functional specifications for the european database on medical devices (eudamed) The. Medical Device List Eu.
From www.greenlight.guru
Ultimate Guide to Device Class Requirements under EU MDR Medical Device List Eu In the european union (eu) they must undergo a conformity. Welcome to the eudamed information centre. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. Medical devices are products or equipment intended for a medical purpose. Functional specifications for the european database on medical devices (eudamed) The european medical device nomenclature (emdn) is the nomenclature. Medical Device List Eu.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I Medical Device List Eu Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. Functional specifications for the european database on medical devices (eudamed) The eu revised the laws governing medical devices and in vitro diagnostics to align with. Medical Device List Eu.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Medical Device List Eu Medical devices are products or equipment intended for a medical purpose. The central point of support for eudamed users, presenting action steps and process logic from. In the european union (eu) they must undergo a conformity. Welcome to the eudamed information centre. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. The european. Medical Device List Eu.
From smartdataweek.com
Medical Device Classification (FDA & EU MDR) SimplerQMS (2024) Medical Device List Eu Medical devices are products or equipment intended for a medical purpose. Welcome to the eudamed information centre. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. Functional specifications for the european database on. Medical Device List Eu.
From www.ondrugdelivery.com
THE NEW EU MEDICAL DEVICE REGULATIONS IMPLICATIONS FOR INHALATION Medical Device List Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. In the european union (eu) they must undergo a conformity. The central point of support for eudamed users, presenting action steps and process logic. Medical Device List Eu.
From easymedicaldevice.com
Complete Guide Medical Device Classification EU MDR (Free PDF) Medical Device List Eu The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. The central point of support for eudamed users, presenting action steps and process logic from.. Medical Device List Eu.
From www.researchgate.net
4 List of the European directives regulating medical devices Medical Device List Eu The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. The european medical device nomenclature (emdn) is the nomenclature of use by manufacturers when registering their. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.. Medical Device List Eu.
From www.freyrsolutions.com
Medical Devices and CE Marking Process under the EU MDR Freyr Medical Device List Eu Functional specifications for the european database on medical devices (eudamed) Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. The european commission published implementing decision (eu) 2019/939, designating four entities to assign unique. In the european union (eu). Medical Device List Eu.