Bond Between Table Salt . Describe the energetics of ionic bond formation and breakage. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather than from the electron sharing seen in covalent bonds. A salt molecule contains atoms of two elements: Table salt is an ionic compound, which breaks into its component ions or dissociates in water. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. These atoms arrange themselves into. The molecular formula of table salt—sodium chloride—is nacl. Usually, the structure of table salt is orderly and neat. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Sodium chloride (nacl), widely recognized as table salt, is an ionic compound formed by the combination of sodium and chlorine ions. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: It is essential for life, playing a crucial role in.
from edukar.in
A salt molecule contains atoms of two elements: Table salt is an ionic compound, which breaks into its component ions or dissociates in water. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather than from the electron sharing seen in covalent bonds. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. These atoms arrange themselves into. The molecular formula of table salt—sodium chloride—is nacl. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. Sodium chloride (nacl), widely recognized as table salt, is an ionic compound formed by the combination of sodium and chlorine ions. Describe the energetics of ionic bond formation and breakage.
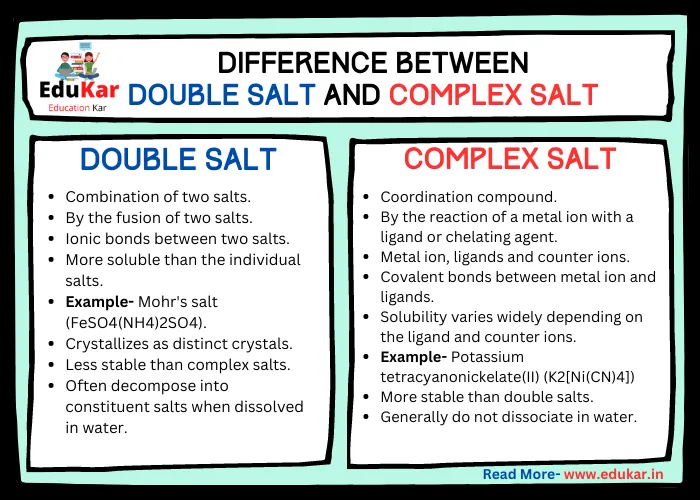
Difference between Double Salt and Complex Salt Edukar India
Bond Between Table Salt These atoms arrange themselves into. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather than from the electron sharing seen in covalent bonds. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Describe the energetics of ionic bond formation and breakage. The molecular formula of table salt—sodium chloride—is nacl. It is essential for life, playing a crucial role in. These atoms arrange themselves into. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. Sodium chloride (nacl), widely recognized as table salt, is an ionic compound formed by the combination of sodium and chlorine ions. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: A salt molecule contains atoms of two elements: The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. Usually, the structure of table salt is orderly and neat.
From elchoroukhost.net
Difference Between Sea Salt And Table Salt Elcho Table Bond Between Table Salt These atoms arrange themselves into. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here,. Bond Between Table Salt.
From www.youtube.com
Sea Salt vs. Table Salt. What's the Difference Between Table Salt and Bond Between Table Salt Usually, the structure of table salt is orderly and neat. The molecular formula of table salt—sodium chloride—is nacl. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. It is essential for life, playing a crucial role in. The sodium and chlorine. Bond Between Table Salt.
From www.sciencephoto.com
Table salt and its molecular model Stock Image C019/8330 Science Bond Between Table Salt These atoms arrange themselves into. The molecular formula of table salt—sodium chloride—is nacl. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather than from the electron sharing seen in covalent bonds. Compounds composed of ions are called. Bond Between Table Salt.
From www.erinnudi.com
Difference between table salt and sea salt Bond Between Table Salt The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between. Bond Between Table Salt.
From niftyrecipe.com
Celtic Salt vs. Table Salt Uncovering the Key Differences and Benefits Bond Between Table Salt These atoms arrange themselves into. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Describe the energetics of ionic bond formation and breakage. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom. Bond Between Table Salt.
From awesomehome.co
Kosher Salt Vs Table Ratio Awesome Home Bond Between Table Salt These atoms arrange themselves into. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. Usually, the structure of table salt is orderly and neat. A salt molecule contains atoms of two elements: Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from. Bond Between Table Salt.
From www.mashed.com
The Real Difference Between Table Salt And Sea Salt Bond Between Table Salt Sodium chloride (nacl), widely recognized as table salt, is an ionic compound formed by the combination of sodium and chlorine ions. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather than from the electron sharing seen in. Bond Between Table Salt.
From www.saltwest.ca
What's Difference Between Sea Salt & Table Salt? Saltwest Naturals Inc Bond Between Table Salt A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: A salt molecule contains atoms of two elements: Table salt is an. Bond Between Table Salt.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Bond Between Table Salt Describe the energetics of ionic bond formation and breakage. Sodium chloride (nacl), widely recognized as table salt, is an ionic compound formed by the combination of sodium and chlorine ions. Usually, the structure of table salt is orderly and neat. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation. Bond Between Table Salt.
From handletheheat.com
Kosher Salt vs. Sea Salt vs. Table Salt Handle the Heat Bond Between Table Salt A salt molecule contains atoms of two elements: Sodium chloride (nacl), widely recognized as table salt, is an ionic compound formed by the combination of sodium and chlorine ions. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. It is essential. Bond Between Table Salt.
From www.pinterest.com
Sea Salt vs. Table Salt Table salt, Nutritional therapy association Bond Between Table Salt A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Describe the energetics of ionic bond formation and breakage. Usually, the structure of table salt is orderly and neat. Compounds composed of ions are called ionic compounds (or salts), and their constituent. Bond Between Table Salt.
From www.eatingwell.com
Sea Salt vs. Table Salt What's the Difference? Bond Between Table Salt The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: A salt molecule contains atoms of two elements: The molecular formula of table salt—sodium chloride—is nacl. A bond’s strength describes how. Bond Between Table Salt.
From himalayansalthub.com
Celtic Salt vs Himalayan Salt Battle of the Salts (2023) Bond Between Table Salt Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather than from the electron sharing seen in covalent bonds. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice.. Bond Between Table Salt.
From www.momswhothink.com
Iodized Salt vs Salt Key Differences Bond Between Table Salt Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The molecular formula of table salt—sodium chloride—is nacl. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. These atoms arrange themselves into. A bond’s strength describes how strongly each atom. Bond Between Table Salt.
From handletheheat.com
Kosher Salt vs. Sea Salt vs. Table Salt Handle the Heat Bond Between Table Salt The molecular formula of table salt—sodium chloride—is nacl. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. It is essential for life, playing a crucial role in. These atoms arrange themselves into. Usually, the structure of table salt is orderly and neat. A bond’s strength describes how strongly each atom. Bond Between Table Salt.
From www.researchgate.net
Salt bridge bonds between IMM01 and CD47. Download Scientific Diagram Bond Between Table Salt Describe the energetics of ionic bond formation and breakage. These atoms arrange themselves into. It is essential for life, playing a crucial role in. The molecular formula of table salt—sodium chloride—is nacl. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. Table salt (sodium chloride, or nacl) is an ionic. Bond Between Table Salt.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Bond Between Table Salt Usually, the structure of table salt is orderly and neat. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another. Bond Between Table Salt.
From differencebetweenz.com
Difference between Table Salt and Kosher Salt Difference Betweenz Bond Between Table Salt It is essential for life, playing a crucial role in. Sodium chloride (nacl), widely recognized as table salt, is an ionic compound formed by the combination of sodium and chlorine ions. Usually, the structure of table salt is orderly and neat. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice.. Bond Between Table Salt.
From www.snexplores.org
Explainer What are chemical bonds? Bond Between Table Salt Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. A salt molecule contains atoms of two elements: The sodium and chlorine. Bond Between Table Salt.
From joisymdal.blob.core.windows.net
How To Make Water Ionic Bonding at Patricia Durst blog Bond Between Table Salt It is essential for life, playing a crucial role in. The molecular formula of table salt—sodium chloride—is nacl. Describe the energetics of ionic bond formation and breakage. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Table salt (sodium chloride, or. Bond Between Table Salt.
From cookingtipoftheday.blogspot.com
Cooking Tip of the Day Salts Sea Salt, Kosher Salt, Table Salt and More Bond Between Table Salt Usually, the structure of table salt is orderly and neat. It is essential for life, playing a crucial role in. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Table salt is an ionic compound, which breaks into its component ions. Bond Between Table Salt.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Bond Between Table Salt The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. It is essential for life, playing a crucial role in. Describe the energetics of ionic bond formation and breakage. The molecular formula of table salt—sodium chloride—is nacl. A bond’s strength describes how strongly each atom is joined to another atom, and. Bond Between Table Salt.
From www.colourbox.com
The process of dissociation of table salt (sodium chloride) in water Bond Between Table Salt Usually, the structure of table salt is orderly and neat. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. Describe the energetics of ionic bond formation and breakage. These atoms arrange themselves into. Sodium chloride (nacl), widely recognized as table salt, is an ionic compound formed by the combination of sodium and chlorine. Bond Between Table Salt.
From readingandwritingprojectcom.web.fc2.com
the bond in table salt (nacl) is Bond Between Table Salt A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. It is essential for life, playing a crucial role in. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. These atoms arrange. Bond Between Table Salt.
From sphweb.bumc.bu.edu
Chemical Elements Atoms Bond Between Table Salt A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. Describe the energetics of ionic bond formation and breakage. Usually, the structure of. Bond Between Table Salt.
From cabinet.matttroy.net
Is Table Salt A Molecule Or Compound Matttroy Bond Between Table Salt The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. These atoms arrange themselves into. The molecular formula of table salt—sodium chloride—is nacl. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms.. Bond Between Table Salt.
From cartoondealer.com
Sodium Chloride Rock Salt, Halite, Table Salt, Chemical Structure Bond Between Table Salt Describe the energetics of ionic bond formation and breakage. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. It is essential for life, playing a crucial role in. The molecular formula of table salt—sodium. Bond Between Table Salt.
From ceyaznjs.blob.core.windows.net
Does Table Salt Have Ionic Bond at James Contreras blog Bond Between Table Salt A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms. Bond Between Table Salt.
From www.thoughtco.com
Chemical Composition of Table Salt Bond Between Table Salt It is essential for life, playing a crucial role in. A salt molecule contains atoms of two elements: Describe the energetics of ionic bond formation and breakage. The molecular formula of table salt—sodium chloride—is nacl. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between. Bond Between Table Salt.
From pediaa.com
Difference Between Salt and Sodium Definition, Chemical Properties Bond Between Table Salt The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. Describe the energetics of ionic bond formation and breakage. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms. Bond Between Table Salt.
From www.tffn.net
Is Table Salt a Mineral? Exploring the Nature and Benefits of the Bond Between Table Salt The molecular formula of table salt—sodium chloride—is nacl. These atoms arrange themselves into. A salt molecule contains atoms of two elements: Describe the energetics of ionic bond formation and breakage. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. A bond’s strength describes how strongly each atom is joined to another atom, and. Bond Between Table Salt.
From edukar.in
Difference between Double Salt and Complex Salt Edukar India Bond Between Table Salt Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Table salt is an ionic compound, which breaks into its component ions or dissociates in water. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond. Bond Between Table Salt.
From chefd.com
Sea Salt vs Table Salt Discover the Key Differences Bond Between Table Salt The sodium and chlorine atoms are present in equal amounts (1:1 ratio), arranged to form a cubic crystal lattice. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The molecular formula of table salt—sodium chloride—is nacl. A salt molecule contains atoms of two elements: Table salt (sodium chloride, or. Bond Between Table Salt.
From www.thoughtco.com
Chemical Composition of Table Salt Bond Between Table Salt Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: It is essential for life, playing a crucial role in. Sodium chloride (nacl), widely recognized as table salt, is an ionic compound formed by the combination of sodium and chlorine ions. The molecular formula of table salt—sodium chloride—is nacl. Usually,. Bond Between Table Salt.
From www.slideserve.com
PPT Creating Compounds PowerPoint Presentation, free download ID Bond Between Table Salt A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Describe the energetics of ionic bond formation and breakage. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: A salt molecule. Bond Between Table Salt.