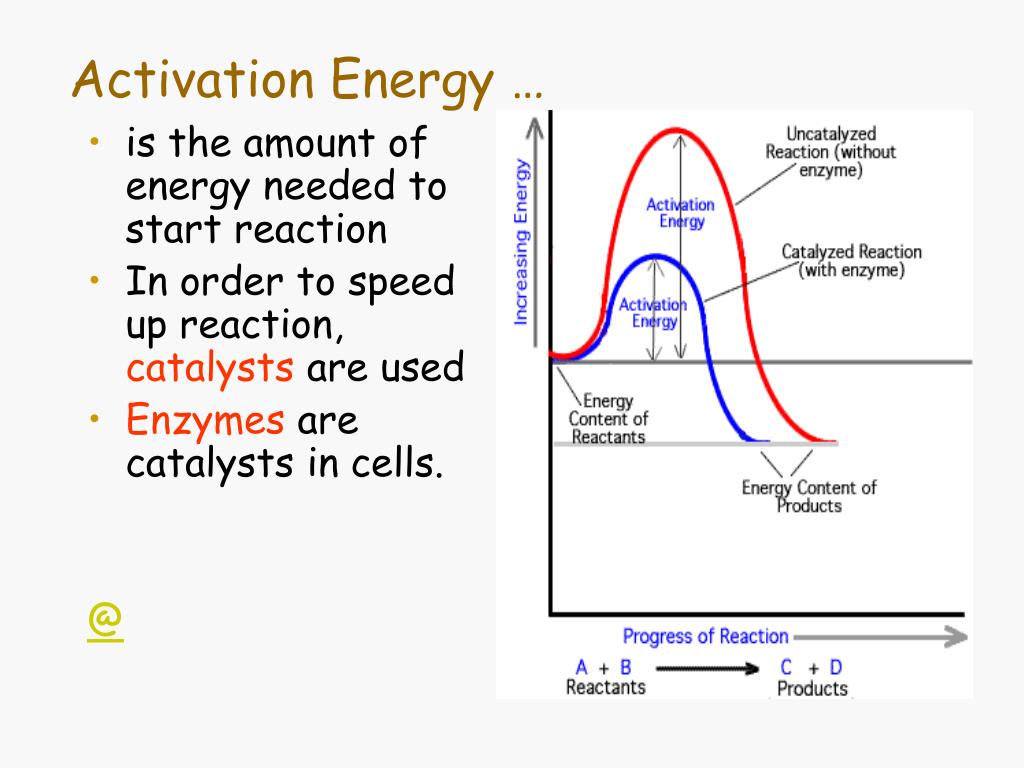
Change The Activation Energy Needed . This is called the activation energy (e a). The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. Reactions require an input of energy to initiate the reaction; Activation energy is the amount of energy. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. The activation energy can be thought of as the magnitude of a potential barrier that the. Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals.
from www.slideserve.com
Activation energy is the amount of energy. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. This is called the activation energy (e a). Reactions require an input of energy to initiate the reaction; Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. The activation energy can be thought of as the magnitude of a potential barrier that the.
PPT Activation Energy … PowerPoint Presentation, free download ID
Change The Activation Energy Needed Reactions require an input of energy to initiate the reaction; Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. The activation energy can be thought of as the magnitude of a potential barrier that the. This is called the activation energy (e a). The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. Reactions require an input of energy to initiate the reaction; Activation energy is the amount of energy. Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1940469 Change The Activation Energy Needed In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. Activation energy is the amount of energy. The activation energy can be thought of as the magnitude of a potential barrier that the. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. Activation energy. Change The Activation Energy Needed.
From www.slideserve.com
PPT Chemical Reactions and Enzymes PowerPoint Presentation, free Change The Activation Energy Needed The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. The activation energy can be thought of as the magnitude of a potential barrier that the. Reactions require an input of energy to initiate the reaction; Even exergonic (spontaneous) reactions typically require a small increase in free energy before. Change The Activation Energy Needed.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Change The Activation Energy Needed Activation energy is the amount of energy. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between. Change The Activation Energy Needed.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Change The Activation Energy Needed Activation energy is the amount of energy. Reactions require an input of energy to initiate the reaction; The activation energy can be thought of as the magnitude of a potential barrier that the. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. Even exergonic (spontaneous) reactions typically require a. Change The Activation Energy Needed.
From www.logiota.com
Activation Energy Chemical Physical Chemistry Chemistry Change The Activation Energy Needed The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. This is called the activation energy (e a). Activation energy (ea) is the minimum amount of energy that must. Change The Activation Energy Needed.
From www.expii.com
Activation Energy — Definition & Importance Expii Change The Activation Energy Needed The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. Activation energy is the amount of energy. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. Reactions require an input of energy to initiate the reaction; In chemistry, activation. Change The Activation Energy Needed.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Change The Activation Energy Needed The activation energy can be thought of as the magnitude of a potential barrier that the. This is called the activation energy (e a). Reactions require an input of energy to initiate the reaction; The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. The initial increase in energy represents. Change The Activation Energy Needed.
From pasqualefifi.blogspot.com
label this diagram energy reaction progress PasqualeFifi Change The Activation Energy Needed Reactions require an input of energy to initiate the reaction; The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. The activation energy. Change The Activation Energy Needed.
From slideplayer.com
CHANGING MATTER Living things use different chemical reactions to get Change The Activation Energy Needed Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the. Change The Activation Energy Needed.
From www.slideserve.com
PPT CHAPTER 3 PowerPoint Presentation, free download ID1361511 Change The Activation Energy Needed Activation energy is the amount of energy. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. This is called the activation energy (e a). In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. The activation energy can be thought of as the. Change The Activation Energy Needed.
From slideplayer.com
Section 2 Chemical Reactions ppt download Change The Activation Energy Needed Activation energy is the amount of energy. Reactions require an input of energy to initiate the reaction; The activation energy can be thought of as the magnitude of a potential barrier that the. Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. Even. Change The Activation Energy Needed.
From design1systems.com
The Role of Activation Energy in Potential Energy Diagrams Change The Activation Energy Needed The activation energy can be thought of as the magnitude of a potential barrier that the. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. Activation energy is the. Change The Activation Energy Needed.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Change The Activation Energy Needed Reactions require an input of energy to initiate the reaction; The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. The activation energy can be thought of as the magnitude of a potential barrier that. Change The Activation Energy Needed.
From www.slideserve.com
PPT Activation Energy … PowerPoint Presentation, free download ID Change The Activation Energy Needed Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. Even exergonic (spontaneous) reactions typically require a small increase in free energy before. Change The Activation Energy Needed.
From opencurriculum.org
Chemical Reactions ‹ OpenCurriculum Change The Activation Energy Needed The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so. Change The Activation Energy Needed.
From slideplayer.com
Enzymes. ppt download Change The Activation Energy Needed In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. The activation energy (\. Change The Activation Energy Needed.
From www.slideserve.com
PPT Chapter 4 The Study of Chemical Reactions PowerPoint Presentation Change The Activation Energy Needed The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to. Change The Activation Energy Needed.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Change The Activation Energy Needed Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. Reactions require an input of energy to initiate the reaction; This is called the activation energy (e a). The activation energy can be thought of as the magnitude of a potential barrier that the.. Change The Activation Energy Needed.
From scienceinfo.com
Activation Energy Definition, Unit, Formula, Calculations Change The Activation Energy Needed The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. This is called the activation energy (e a). Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. In chemistry, activation energy is. Change The Activation Energy Needed.
From general.chemistrysteps.com
Activation Energy Chemistry Steps Change The Activation Energy Needed This is called the activation energy (e a). In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. Activation energy is the amount of energy. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. Even exergonic (spontaneous) reactions typically require a small increase. Change The Activation Energy Needed.
From www.slideserve.com
PPT The Nature of Chemical Reactions PowerPoint Presentation, free Change The Activation Energy Needed This is called the activation energy (e a). The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. Reactions require an input of energy to initiate the reaction; Activation energy is the amount of energy. Activation energy (ea) is the minimum amount of energy that must be provided to. Change The Activation Energy Needed.
From professional.patrickneyman.com
Activation Energy Equation Change The Activation Energy Needed Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. The activation energy can be thought of as the magnitude of a potential barrier that the. Activation energy is. Change The Activation Energy Needed.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation ID239015 Change The Activation Energy Needed The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. The activation energy can be thought of as the magnitude of a potential barrier that the. Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and. Change The Activation Energy Needed.
From brainly.in
Describe how you can determine the total change in enthalpy and Change The Activation Energy Needed The activation energy can be thought of as the magnitude of a potential barrier that the. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. Activation energy is the. Change The Activation Energy Needed.
From bio.libretexts.org
3.9 Energy in Chemical Reactions Biology LibreTexts Change The Activation Energy Needed Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. This is called the activation energy (e a). Activation energy is the amount of energy. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the. Change The Activation Energy Needed.
From www.researchgate.net
Schematic for activation energy of high energetic systems; sufficient Change The Activation Energy Needed Reactions require an input of energy to initiate the reaction; Activation energy is the amount of energy. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. This is. Change The Activation Energy Needed.
From blogs.glowscotland.org.uk
Activation Energy Higher Chemistry Unit 1 Change The Activation Energy Needed The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. Activation energy is the amount of energy. The activation energy can be thought of as the magnitude of a potential barrier that the. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding. Change The Activation Energy Needed.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Change The Activation Energy Needed Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. The activation energy can be thought of as the magnitude of a potential barrier that the. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles. Change The Activation Energy Needed.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Change The Activation Energy Needed Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products. Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. The initial increase in energy represents the activation energy (ea), which is the minimum. Change The Activation Energy Needed.
From douglasnewssantana.blogspot.com
Activation Energy Can Be Described as Change The Activation Energy Needed Activation energy (ea) is the minimum amount of energy that must be provided to reactant molecules or particles so that they can react and become chemicals. In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. This is called the activation energy (e a). Reactions require an input of energy to initiate the reaction; The. Change The Activation Energy Needed.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Change The Activation Energy Needed The activation energy can be thought of as the magnitude of a potential barrier that the. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. Activation energy is the amount of energy. Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin. Change The Activation Energy Needed.
From www.nagwa.com
Question Video Identifying the Activation Energy of an Enzyme Change The Activation Energy Needed The activation energy can be thought of as the magnitude of a potential barrier that the. The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. This is called the activation energy (e a). In chemistry, activation energy is the minimum amount of energy required for a chemical reaction.. Change The Activation Energy Needed.
From socratic.org
What are activation energies? Socratic Change The Activation Energy Needed The initial increase in energy represents the activation energy (ea), which is the minimum energy that colliding particles must have in. Activation energy is the amount of energy. The activation energy can be thought of as the magnitude of a potential barrier that the. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy. Change The Activation Energy Needed.
From fixportillobadgered.z21.web.core.windows.net
Energy Diagram Activation Energy Change The Activation Energy Needed In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. Activation energy is the amount of energy. This is called the activation energy (e a). Reactions require an input of energy to initiate the reaction; Even exergonic (spontaneous) reactions typically require a small increase in free energy before they can begin converting reactants to products.. Change The Activation Energy Needed.
From www.britannica.com
Transitionstate theory Definition & Facts Britannica Change The Activation Energy Needed In chemistry, activation energy is the minimum amount of energy required for a chemical reaction. This is called the activation energy (e a). The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants. Activation energy is the amount of energy. Even exergonic (spontaneous) reactions typically require a small increase in. Change The Activation Energy Needed.