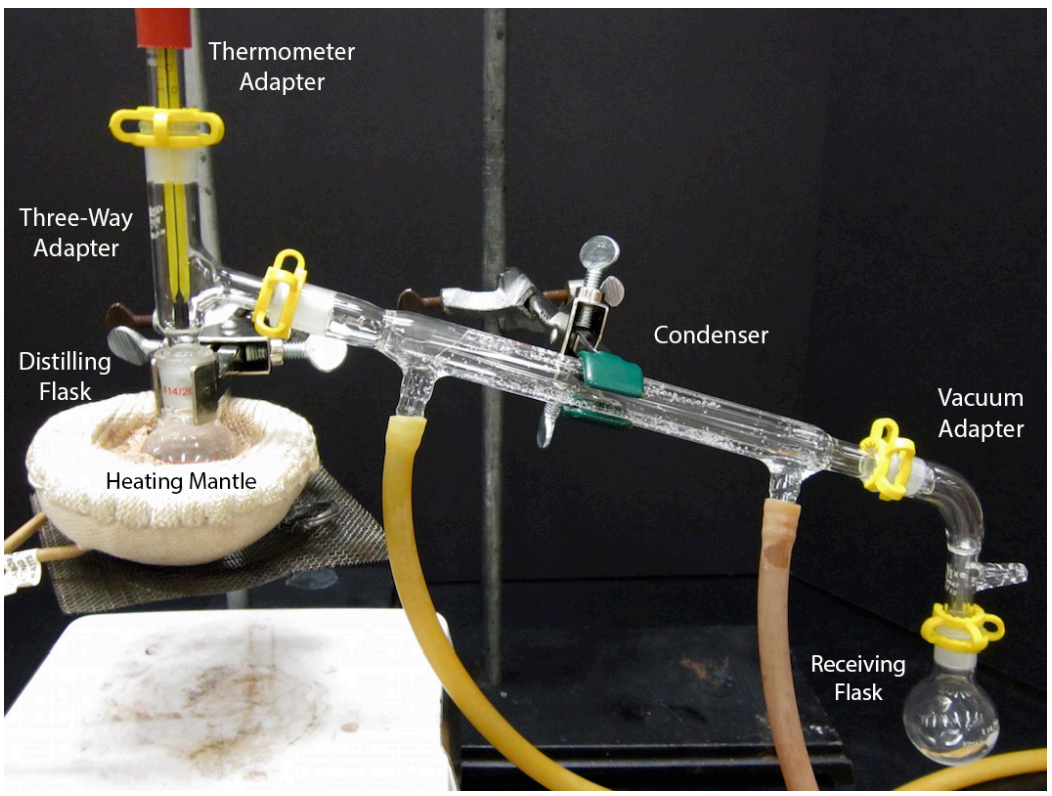
Distillation Chemistry Lab . Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Begin distillation turn on the condenser water. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the It is also used for the separation of mixtures of liquids miscible that differ in b.p. In at least 25 °c. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Simple distillation (the procedure outlined below) can be used effectively to. Distillation is an important commercial process that is used in the purification of a large variety of materials. Simple distillation is a procedure by which two liquids with different boiling points can be separated.
from chem.libretexts.org
Begin distillation turn on the condenser water. Simple distillation is a procedure by which two liquids with different boiling points can be separated. Collect distillate at a rate of 1 drop per second. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Distillation is an important commercial process that is used in the purification of a large variety of materials. Apply the heat source to the distilling flask. Record the temperature where liquid is actively distilling and thermometer bulb is immersed.
5.2C StepbyStep Procedures Chemistry LibreTexts
Distillation Chemistry Lab Apply the heat source to the distilling flask. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Begin distillation turn on the condenser water. Simple distillation is a procedure by which two liquids with different boiling points can be separated. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. It is also used for the separation of mixtures of liquids miscible that differ in b.p. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Apply the heat source to the distilling flask. Simple distillation (the procedure outlined below) can be used effectively to. Collect distillate at a rate of 1 drop per second. Distillation is an important commercial process that is used in the purification of a large variety of materials. In at least 25 °c. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids.
From chem.libretexts.org
5.3A Theory of Fractional Distillation Chemistry LibreTexts Distillation Chemistry Lab It is also used for the separation of mixtures of liquids miscible that differ in b.p. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. Collect distillate at a rate of 1 drop per second. Simple distillation (the procedure outlined below) can be used effectively to. Distillation is a purification method for. Distillation Chemistry Lab.
From www.aliexpress.com
Chemistry 1000ml Distillation Apparatus Laboratory Glassware Kit Set Distillation Chemistry Lab Collect distillate at a rate of 1 drop per second. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Apply the heat source to the distilling flask. Begin distillation turn on the condenser water. Distillation is one of the oldest and still most common methods for both the purification and. Distillation Chemistry Lab.
From www.shutterstock.com
Vektor Stok Simple Distillation Laboratory Set Separation Homogeneous Distillation Chemistry Lab In at least 25 °c. Apply the heat source to the distilling flask. Simple distillation (the procedure outlined below) can be used effectively to. Simple distillation is a procedure by which two liquids with different boiling points can be separated. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Collect. Distillation Chemistry Lab.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Distillation Chemistry Lab Simple distillation is a procedure by which two liquids with different boiling points can be separated. In at least 25 °c. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Distillation is a purification method. Distillation Chemistry Lab.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Distillation Chemistry Lab However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Simple distillation is a procedure by which two liquids with different boiling points can be separated. Distillation is an important commercial process that is used in the purification of a large variety of materials. Simple distillation (the procedure outlined below). Distillation Chemistry Lab.
From www.pinterest.com
Fractional Distillation Vector Illustration Labeled Educational Stock Distillation Chemistry Lab Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Collect distillate at a rate of 1 drop per second. It is also used for the separation of mixtures of liquids miscible that differ in b.p. However, before we begin a discussion of distillation, it would probably be beneficial. Distillation Chemistry Lab.
From chem.libretexts.org
5.5D StepbyStep Procedures for Steam Distillation Chemistry LibreTexts Distillation Chemistry Lab Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Collect distillate at a rate of 1 drop per second. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. It is also used for the separation of mixtures of liquids miscible that differ in b.p. Distillation is an important. Distillation Chemistry Lab.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Distillation Chemistry Lab Apply the heat source to the distilling flask. In at least 25 °c. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Distillation is an important commercial process that is used in the purification of a. Distillation Chemistry Lab.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Distillation Chemistry Lab Simple distillation is a procedure by which two liquids with different boiling points can be separated. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Begin distillation turn on the condenser. Distillation Chemistry Lab.
From www.amazon.co.uk
New DIY Distillation Unit 500ml Lab Glassware Kit Chemistry Labware Set Distillation Chemistry Lab Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Distillation is an important commercial process that is used in the purification of a large variety of materials. Apply the heat source to the distilling flask. Record the temperature where liquid is actively distilling and thermometer bulb is immersed.. Distillation Chemistry Lab.
From www.thoughtco.com
What Is Distillation? Principles and Uses Distillation Chemistry Lab In at least 25 °c. It is also used for the separation of mixtures of liquids miscible that differ in b.p. Begin distillation turn on the condenser water. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Simple distillation is a procedure by which two liquids with different. Distillation Chemistry Lab.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Distillation Chemistry Lab Distillation is an important commercial process that is used in the purification of a large variety of materials. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Apply the heat source to the distilling flask. However, before we begin a discussion of distillation, it would probably be beneficial to define. Distillation Chemistry Lab.
From www.vernier.com
Fractional Distillation > Experiment 8 from Chemistry with Vernier Distillation Chemistry Lab However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Simple distillation is a procedure by which two liquids with different boiling points can be separated. Apply the heat source to the distilling flask. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Mixtures can be. Distillation Chemistry Lab.
From www.circuitdiagram.co
Schematic Diagram Of Simple Distillation Experiment Circuit Diagram Distillation Chemistry Lab In at least 25 °c. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Simple distillation (the procedure outlined below) can be used effectively to. Distillation is an important commercial process that is used in the purification of a large variety of materials. It is also used for the separation of mixtures of liquids miscible that. Distillation Chemistry Lab.
From tnlab.com
Short Path Distillation System 20 Liter TN LAB Supply Distillation Chemistry Lab Simple distillation is a procedure by which two liquids with different boiling points can be separated. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Distillation is an important commercial process that is used in the purification of a large variety of materials. Record the temperature where liquid is. Distillation Chemistry Lab.
From www.aliexpress.com
Laboratory Equipment 20L Short Path Distillation Chemistry Lab Distillation Chemistry Lab Begin distillation turn on the condenser water. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Collect distillate at a rate of 1 drop per second. It is also used for the separation of mixtures of liquids miscible that differ in b.p. Distillation is a purification method for liquids,. Distillation Chemistry Lab.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Distillation Chemistry Lab Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Begin distillation turn on the condenser water. It is also used for the separation of mixtures of liquids miscible that differ in b.p. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that. Distillation Chemistry Lab.
From chem.libretexts.org
5.3D StepbyStep Procedures for Fractional Distillation Chemistry Distillation Chemistry Lab Distillation is an important commercial process that is used in the purification of a large variety of materials. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Simple distillation is. Distillation Chemistry Lab.
From chem.libretexts.org
5.2C StepbyStep Procedures Chemistry LibreTexts Distillation Chemistry Lab Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Simple distillation is a procedure by which two liquids with different boiling points can be separated. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Begin distillation turn on the. Distillation Chemistry Lab.
From en.wikipedia.org
Distillation Wikipedia Distillation Chemistry Lab However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Apply the heat source to the distilling flask. Simple distillation (the procedure outlined below) can be used effectively to. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. Collect distillate at a. Distillation Chemistry Lab.
From socratic.org
How can compounds in a mixture be separated? Socratic Distillation Chemistry Lab It is also used for the separation of mixtures of liquids miscible that differ in b.p. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Distillation is an important commercial process that is used in the purification of a large variety of materials. Simple distillation (the procedure outlined below) can. Distillation Chemistry Lab.
From www.scientificglassservices.co.uk
Selecting Laboratory Glassware for Distillation Scientific Glass Services Distillation Chemistry Lab Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. Apply the heat source to the distilling flask. In at least 25 °c. Simple distillation is a procedure by which two liquids with different boiling points can be separated. It is also used for the separation of mixtures of liquids miscible that differ. Distillation Chemistry Lab.
From www.freepik.com
Premium Vector Simple distillation model in chemistry laboratory vector Distillation Chemistry Lab It is also used for the separation of mixtures of liquids miscible that differ in b.p. Simple distillation (the procedure outlined below) can be used effectively to. Collect distillate at a rate of 1 drop per second. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Simple distillation is a. Distillation Chemistry Lab.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Distillation Chemistry Lab Distillation is an important commercial process that is used in the purification of a large variety of materials. It is also used for the separation of mixtures of liquids miscible that differ in b.p. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. However, before we begin a. Distillation Chemistry Lab.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Distillation Chemistry Lab Begin distillation turn on the condenser water. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. It is also used for the separation of mixtures of liquids miscible that differ in b.p. Collect. Distillation Chemistry Lab.
From www.pinterest.com
Pin by Team Distillation on Distillation Distillation, Distillation Distillation Chemistry Lab Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Simple distillation (the procedure outlined below) can be used effectively to. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Simple distillation is a procedure by which two liquids with different boiling points can be. Distillation Chemistry Lab.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Distillation Chemistry Lab Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Simple distillation is a procedure by which two liquids with different boiling points can be separated. In at least 25 °c. Mixtures can be separated by physical processes. Distillation Chemistry Lab.
From byjus.com
Refining Process Against Impurities Oil Refining Process Chemistry Distillation Chemistry Lab Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. It is also used for the separation of mixtures of liquids miscible that differ in b.p. Begin distillation turn on the condenser water. In at least 25 °c. Distillation is one of the oldest and still most common methods for both the purification. Distillation Chemistry Lab.
From www.aliexpress.com
2L Short Path Distillation, Essential Oil Distillation Equipment Distillation Chemistry Lab Collect distillate at a rate of 1 drop per second. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. Apply the heat source to the distilling flask. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. However, before we begin a discussion of distillation, it would. Distillation Chemistry Lab.
From www.shutterstock.com
530 Fractional distillation 이미지, 스톡 사진 및 벡터 Shutterstock Distillation Chemistry Lab Simple distillation (the procedure outlined below) can be used effectively to. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Simple distillation is a procedure by which two liquids with different boiling points can be separated. Distillation is a purification method for liquids, and can separate components of. Distillation Chemistry Lab.
From glossary.periodni.com
Distillation Chemistry Dictionary & Glossary Distillation Chemistry Lab Collect distillate at a rate of 1 drop per second. Simple distillation (the procedure outlined below) can be used effectively to. Record the temperature where liquid is actively distilling and thermometer bulb is immersed. It is also used for the separation of mixtures of liquids miscible that differ in b.p. Begin distillation turn on the condenser water. Apply the heat. Distillation Chemistry Lab.
From www.etsy.com
Distillation Apparatus Diagram Poster Printable Lab Tools Etsy Distillation Chemistry Lab Simple distillation is a procedure by which two liquids with different boiling points can be separated. It is also used for the separation of mixtures of liquids miscible that differ in b.p. Apply the heat source to the distilling flask. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. Distillation is one. Distillation Chemistry Lab.
From www.shutterstock.com
diagramme de distillation simple en chimie image vectorielle de stock Distillation Chemistry Lab Distillation is an important commercial process that is used in the purification of a large variety of materials. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. Simple distillation (the procedure outlined below) can be used effectively to. Simple distillation is a procedure by which two liquids with different boiling points can. Distillation Chemistry Lab.
From chem.libretexts.org
1A.3 Classifying Matter Chemistry LibreTexts Distillation Chemistry Lab Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. Simple distillation is a procedure by which two liquids with different boiling points can be separated. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. Simple distillation (the procedure outlined below) can be used. Distillation Chemistry Lab.
From www.vecteezy.com
Distillation Vector Art, Icons, and Graphics for Free Download Distillation Chemistry Lab In at least 25 °c. Collect distillate at a rate of 1 drop per second. However, before we begin a discussion of distillation, it would probably be beneficial to define the terms that describe the Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Distillation is one of the oldest. Distillation Chemistry Lab.