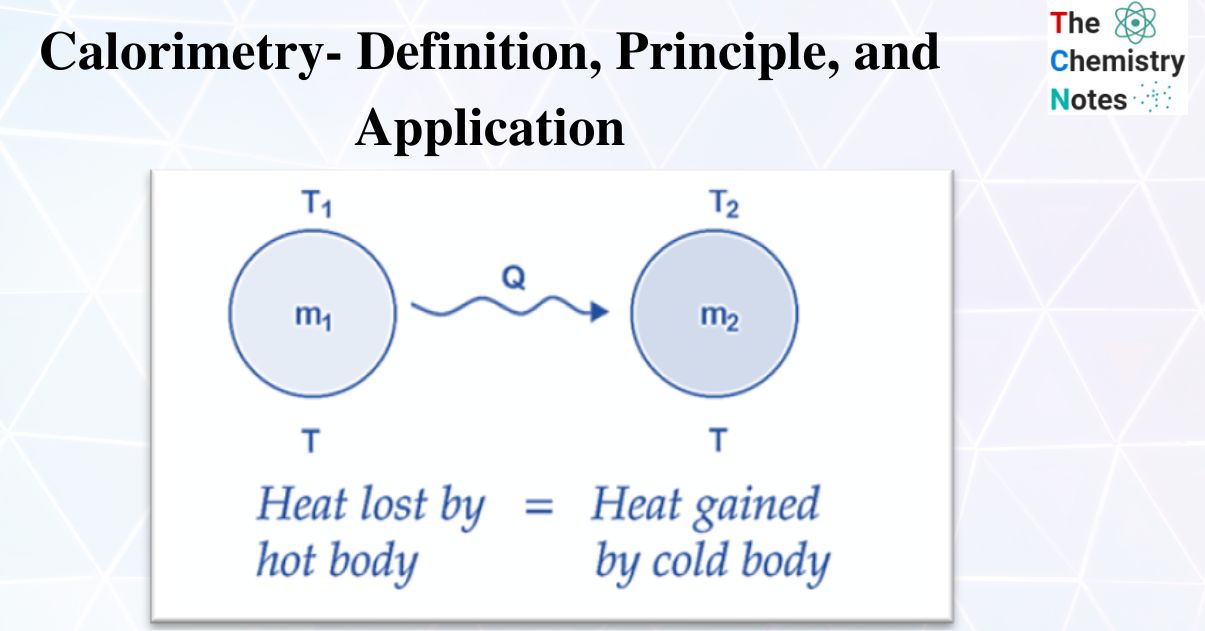
Calorimeter Law Definition . a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The principle of calorimetry indicates the law of conservation energy, i.e. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn how to use the. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. calorimetry is a method to measure heat transfer and enthalpy changes using a thermally isolated device called a. Heat lost = heat gained. learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. calorimetry is used to measure amounts of heat transferred to or from a substance. The total heat lost by the hot body is equal to the total heat gained by the cold body. To do so, the heat is exchanged with a calibrated object. the body at higher temperature releases heat while the body at lower temperature absorbs heat. Learn how to calculate enthalpy changes using.
from thechemistrynotes.com
calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The total heat lost by the hot body is equal to the total heat gained by the cold body. The principle of calorimetry indicates the law of conservation energy, i.e. the body at higher temperature releases heat while the body at lower temperature absorbs heat. Learn how to calculate enthalpy changes using. calorimetry is a method to measure heat transfer and enthalpy changes using a thermally isolated device called a. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters.
Calorimetry Definition, Principle, Types, Application, and Limitations
Calorimeter Law Definition To do so, the heat is exchanged with a calibrated object. learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. the body at higher temperature releases heat while the body at lower temperature absorbs heat. calorimetry is used to measure amounts of heat transferred to or from a substance. The total heat lost by the hot body is equal to the total heat gained by the cold body. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is a method to measure heat transfer and enthalpy changes using a thermally isolated device called a. To do so, the heat is exchanged with a calibrated object. Learn how to calculate enthalpy changes using. Heat lost = heat gained. Learn how to use the. calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. The principle of calorimetry indicates the law of conservation energy, i.e. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From gamesmartz.com
Calorimeter Definition Easy to Understand Calorimeter Law Definition learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. calorimetry is a method to measure heat transfer and enthalpy changes using a thermally isolated device called a. To do so, the heat is exchanged with a calibrated object. calorimetry is a technique to measure enthalpy. Calorimeter Law Definition.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Law Definition calorimetry is a method to measure heat transfer and enthalpy changes using a thermally isolated device called a. the body at higher temperature releases heat while the body at lower temperature absorbs heat. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the. Calorimeter Law Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Law Definition the body at higher temperature releases heat while the body at lower temperature absorbs heat. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. calorimetry is used to measure amounts of heat transferred to or from a substance. Heat lost = heat gained. calorimetry is a technique to measure. Calorimeter Law Definition.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimeter Law Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. calorimetry is a method to measure heat transfer and enthalpy changes using a thermally isolated device called a. Learn how to calculate. Calorimeter Law Definition.
From kaffee.50webs.com
Lab Calorimetry Calorimeter Law Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The principle of calorimetry indicates the law of conservation energy, i.e. Heat lost = heat gained. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The total heat lost. Calorimeter Law Definition.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimeter Law Definition calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to calculate enthalpy changes using. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. calorimetry is a field. Calorimeter Law Definition.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Calorimeter Law Definition learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. Learn how to use the. a calorimeter is a device used to measure the amount of heat involved in a. Calorimeter Law Definition.
From www.slideserve.com
PPT First Law of Thermodynamics (Law of Conservation of Energy Calorimeter Law Definition To do so, the heat is exchanged with a calibrated object. calorimetry is a method to measure heat transfer and enthalpy changes using a thermally isolated device called a. Learn how to calculate enthalpy changes using. Heat lost = heat gained. the body at higher temperature releases heat while the body at lower temperature absorbs heat. calorimetry. Calorimeter Law Definition.
From www.slideserve.com
PPT An introduction to calorimeters for particle physics PowerPoint Calorimeter Law Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. Learn how to use the. the body at higher temperature releases heat while the body at lower. Calorimeter Law Definition.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Law Definition learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. Learn how to use the. The total heat lost by the hot body is equal to the total heat gained by the cold body. Heat lost = heat gained. calorimetry is a technique to measure enthalpy changes in chemical processes using devices. Calorimeter Law Definition.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Law Definition calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. Heat lost = heat gained. To do so, the heat is exchanged with a calibrated object. Learn how to use the. calorimetry is used to measure amounts of heat transferred to or from a substance. The total heat lost by the hot body. Calorimeter Law Definition.
From cebkmocg.blob.core.windows.net
What Is A Bomb Calorimeter Definition at Donna Chaney blog Calorimeter Law Definition calorimetry is a method to measure heat transfer and enthalpy changes using a thermally isolated device called a. learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. the body at higher temperature releases heat while the body at lower temperature absorbs heat. calorimetry is. Calorimeter Law Definition.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Law Definition calorimetry is a method to measure heat transfer and enthalpy changes using a thermally isolated device called a. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. The principle of calorimetry indicates the law of conservation energy,. Calorimeter Law Definition.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID197266 Calorimeter Law Definition The principle of calorimetry indicates the law of conservation energy, i.e. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. the body. Calorimeter Law Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Law Definition the body at higher temperature releases heat while the body at lower temperature absorbs heat. The total heat lost by the hot body is equal to the total heat gained by the cold body. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. calorimetry is a method to measure heat. Calorimeter Law Definition.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimeter Law Definition Heat lost = heat gained. calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to calculate enthalpy changes using. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. the body at higher temperature releases heat while the body at. Calorimeter Law Definition.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Law Definition calorimetry is used to measure amounts of heat transferred to or from a substance. Learn how to use the. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. The principle of calorimetry indicates the law of conservation energy, i.e. Heat lost = heat gained. calorimetry is a field of thermochemistry. Calorimeter Law Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Law Definition Heat lost = heat gained. calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. The total heat lost by the hot body is equal to the total heat gained by the cold body. the body at higher temperature releases heat while the body at lower temperature absorbs heat. The principle of calorimetry. Calorimeter Law Definition.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Law Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The principle of calorimetry indicates the law of conservation energy, i.e. calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. a calorimeter is a device used to measure the amount of heat. Calorimeter Law Definition.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimeter Law Definition the body at higher temperature releases heat while the body at lower temperature absorbs heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. The principle. Calorimeter Law Definition.
From philschatz.com
Calorimetry · Chemistry Calorimeter Law Definition The principle of calorimetry indicates the law of conservation energy, i.e. Learn how to calculate enthalpy changes using. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. the body at higher temperature releases heat while the body. Calorimeter Law Definition.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Law Definition Learn how to calculate enthalpy changes using. The principle of calorimetry indicates the law of conservation energy, i.e. Learn how to use the. To do so, the heat is exchanged with a calibrated object. the body at higher temperature releases heat while the body at lower temperature absorbs heat. The total heat lost by the hot body is equal. Calorimeter Law Definition.
From www.youtube.com
050 Calorimetry YouTube Calorimeter Law Definition Learn how to use the. The total heat lost by the hot body is equal to the total heat gained by the cold body. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. Learn how to calculate enthalpy changes using. To do so, the heat is exchanged with a calibrated object. . Calorimeter Law Definition.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Law Definition Learn how to calculate enthalpy changes using. To do so, the heat is exchanged with a calibrated object. Learn how to use the. The total heat lost by the hot body is equal to the total heat gained by the cold body. calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. calorimetry. Calorimeter Law Definition.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimeter Law Definition calorimetry is used to measure amounts of heat transferred to or from a substance. learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. The total heat lost by the. Calorimeter Law Definition.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimeter Law Definition the body at higher temperature releases heat while the body at lower temperature absorbs heat. Learn how to calculate enthalpy changes using. To do so, the heat is exchanged with a calibrated object. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat lost = heat gained. The. Calorimeter Law Definition.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter Law Definition learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. To do so, the heat is exchanged with a calibrated object. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a technique to measure. Calorimeter Law Definition.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimeter Law Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. the body at higher temperature releases heat while the body at lower temperature absorbs heat. learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. calorimetry. Calorimeter Law Definition.
From quizgrouchiest.z4.web.core.windows.net
Calorimeter Constant Definition Chemistry Calorimeter Law Definition learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The principle of calorimetry indicates the law of conservation energy, i.e. Learn how to use the. To do. Calorimeter Law Definition.
From exopfwwvc.blob.core.windows.net
Calorimeter In Physics Definition at Laura Addy blog Calorimeter Law Definition calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. The total heat lost by the hot body is equal to the total heat gained by the. Calorimeter Law Definition.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Law Definition To do so, the heat is exchanged with a calibrated object. The total heat lost by the hot body is equal to the total heat gained by the cold body. learn what calorimetry is, how it works, and what types of calorimeters are used to measure heat exchange and enthalpy changes. learn how to measure the amount of. Calorimeter Law Definition.
From cebkmocg.blob.core.windows.net
What Is A Bomb Calorimeter Definition at Donna Chaney blog Calorimeter Law Definition To do so, the heat is exchanged with a calibrated object. calorimetry is a technique to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to calculate enthalpy changes using. Heat lost = heat gained. calorimetry is a method to measure heat transfer and enthalpy changes using a thermally isolated device called a. learn. Calorimeter Law Definition.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Calorimeter Law Definition Learn how to calculate enthalpy changes using. To do so, the heat is exchanged with a calibrated object. Heat lost = heat gained. the body at higher temperature releases heat while the body at lower temperature absorbs heat. Learn how to use the. The total heat lost by the hot body is equal to the total heat gained by. Calorimeter Law Definition.
From studylib.net
Calorimetry Calorimeter Law Definition To do so, the heat is exchanged with a calibrated object. The total heat lost by the hot body is equal to the total heat gained by the cold body. learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. Heat lost = heat gained. Learn how to calculate enthalpy changes using. . Calorimeter Law Definition.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID5405762 Calorimeter Law Definition learn how to measure the amount of heat involved in chemical or physical processes using calorimeters. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The principle of. Calorimeter Law Definition.