Iron Quantum Numbers . this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. It has an atomic weight of 55.845. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons.
from www.learninsta.com
learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. It has an atomic weight of 55.845. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material.
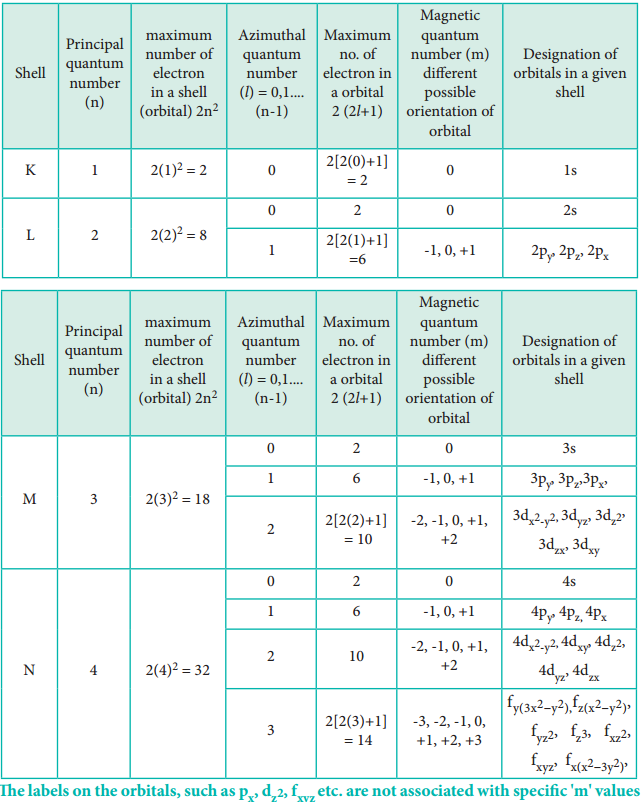
Quantum Numbers
Iron Quantum Numbers having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. It has an atomic weight of 55.845. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal.
From shanimchemistry.blogspot.com
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS Iron Quantum Numbers It has an atomic weight of 55.845. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. iron is the 26th element in the periodic table and has a symbol. Iron Quantum Numbers.
From www.researchgate.net
Principal quantum number, types and number of orbitals, maximum number Iron Quantum Numbers It has an atomic weight of 55.845. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. this section covers some of the more important quantum numbers and. Iron Quantum Numbers.
From study.com
Quantum Numbers on the Periodic Table Definition & Overview Lesson Iron Quantum Numbers this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. learn how to use. Iron Quantum Numbers.
From chemistrypubs.com
Quantum Numbers Principle, Examples, Significance Chemistrupubs Iron Quantum Numbers having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. learn how. Iron Quantum Numbers.
From www.slideserve.com
PPT Quantum Mechanical Model PowerPoint Presentation, free download Iron Quantum Numbers learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. iron. Iron Quantum Numbers.
From www.slideshare.net
Quantum numbers Iron Quantum Numbers learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. It has an atomic weight of 55.845. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. this section covers some of the more important quantum numbers and rules—all of which. Iron Quantum Numbers.
From www.pinterest.com
Quantum Numbers Tutoring Pinterest Number, Chemistry and Physics Iron Quantum Numbers It has an atomic weight of 55.845. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. learn how to use quantum numbers (n, l, and ml) to describe the spatial. Iron Quantum Numbers.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Quantum Numbers this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. It has an atomic weight of 55.845. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. having introduced the basics of atomic structure and quantum mechanics, we can use our. Iron Quantum Numbers.
From isemprole.my.to
Quantum Numbers Principal, Azimuthal, & Spin Iron Quantum Numbers learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26.. Iron Quantum Numbers.
From www.slideserve.com
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation Iron Quantum Numbers learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. It has an atomic weight of 55.845. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution. Iron Quantum Numbers.
From www.slideserve.com
PPT Unit 3 Atomic Theory & Quantum Mechanics Sections A.4 A.5 Iron Quantum Numbers this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. It has an atomic weight of 55.845. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. iron is the 26th element in the periodic table and has a symbol of. Iron Quantum Numbers.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Quantum Numbers It has an atomic weight of 55.845. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. this section covers some of the more important quantum numbers and rules—all of. Iron Quantum Numbers.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps Iron Quantum Numbers learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. It has an atomic weight of 55.845. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons. Iron Quantum Numbers.
From chemistrypubs.com
Quantum Numbers Principle, Examples, Significance Chemistrupubs Iron Quantum Numbers learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. It has an atomic weight of 55.845. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. having introduced the basics of atomic structure and quantum mechanics, we. Iron Quantum Numbers.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Iron Quantum Numbers It has an atomic weight of 55.845. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. iron is the 26th element in the periodic table and has a symbol of. Iron Quantum Numbers.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin Iron Quantum Numbers iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. It has an atomic weight of 55.845. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. learn how the four quantum numbers (principal, secondary, magnetic and spin). Iron Quantum Numbers.
From www.showme.com
How to find elements given quantum numbers ShowMe Iron Quantum Numbers It has an atomic weight of 55.845. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. this section covers some of the more important quantum numbers and rules—all of which. Iron Quantum Numbers.
From www.studocu.com
Quantum numbers of chemistry Homework 9 Quantum Numbers Name Iron Quantum Numbers this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. It has an atomic weight of 55.845. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. iron is the 26th element in the periodic table and has a symbol of fe. Iron Quantum Numbers.
From en.ppt-online.org
Atomic structure and properties. (Chapter 3) online presentation Iron Quantum Numbers learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. learn how to use quantum. Iron Quantum Numbers.
From physicscatalyst.com
Quantum Numbers Chart physicscatalyst's Blog Iron Quantum Numbers iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. It has. Iron Quantum Numbers.
From www.slideserve.com
PPT CHAPTER 7 AND PART 16 PowerPoint Presentation, free download ID Iron Quantum Numbers learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. It has an atomic weight. Iron Quantum Numbers.
From heemskerksnursery.com
Easy Way to Teach Quantum Numbers — CoScine Creative Quantum Numbers Iron Quantum Numbers It has an atomic weight of 55.845. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution. Iron Quantum Numbers.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition Iron Quantum Numbers learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. It has an atomic weight. Iron Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID6766216 Iron Quantum Numbers having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. this section. Iron Quantum Numbers.
From www.sarthaks.com
Match the quantum numbers with the information provided by these Iron Quantum Numbers iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. It has. Iron Quantum Numbers.
From www.slideserve.com
PPT Arrangement of Electrons in Atoms PowerPoint Presentation, free Iron Quantum Numbers learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. . Iron Quantum Numbers.
From www.numerade.com
Give the allowable combinations of quantum numbers for each of the Iron Quantum Numbers having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. learn how quantum mechanics explains the behavior of electrons in atoms using four quantum numbers, including the principal. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. It has an atomic. Iron Quantum Numbers.
From www.learninsta.com
Quantum Numbers Iron Quantum Numbers It has an atomic weight of 55.845. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of. Iron Quantum Numbers.
From mavink.com
Quantum Numbers Symbols Iron Quantum Numbers iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. It has an atomic weight of 55.845. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. learn how to use quantum numbers (n, l, and ml) to describe the. Iron Quantum Numbers.
From www.shutterstock.com
Table Quantum Numbers Stock Vector (Royalty Free) 209972044 Shutterstock Iron Quantum Numbers iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. It has. Iron Quantum Numbers.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition Iron Quantum Numbers this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. learn how to use quantum. Iron Quantum Numbers.
From kunduz.com
[ANSWERED] Write the 4 quantum numbers for iron's L... Iron Quantum Numbers iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. having introduced. Iron Quantum Numbers.
From www.youtube.com
UPSC Preparation Quantum numbers YouTube Iron Quantum Numbers learn how the four quantum numbers (principal, secondary, magnetic and spin) describe the distribution of electrons. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. It has an atomic weight of 55.845. iron is the 26th element in the periodic table and has a symbol of fe. Iron Quantum Numbers.
From www.youtube.com
Quantum Numbers Explained (A fully explained lesson) YouTube Iron Quantum Numbers this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms.. Iron Quantum Numbers.
From www.youtube.com
Quantum numbers and orbitals YouTube Iron Quantum Numbers this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material. learn how to use quantum numbers (n, l, and ml) to describe the spatial distribution of electrons in atoms. iron is the 26th element in the periodic table and has a symbol of fe and atomic number of 26.. Iron Quantum Numbers.