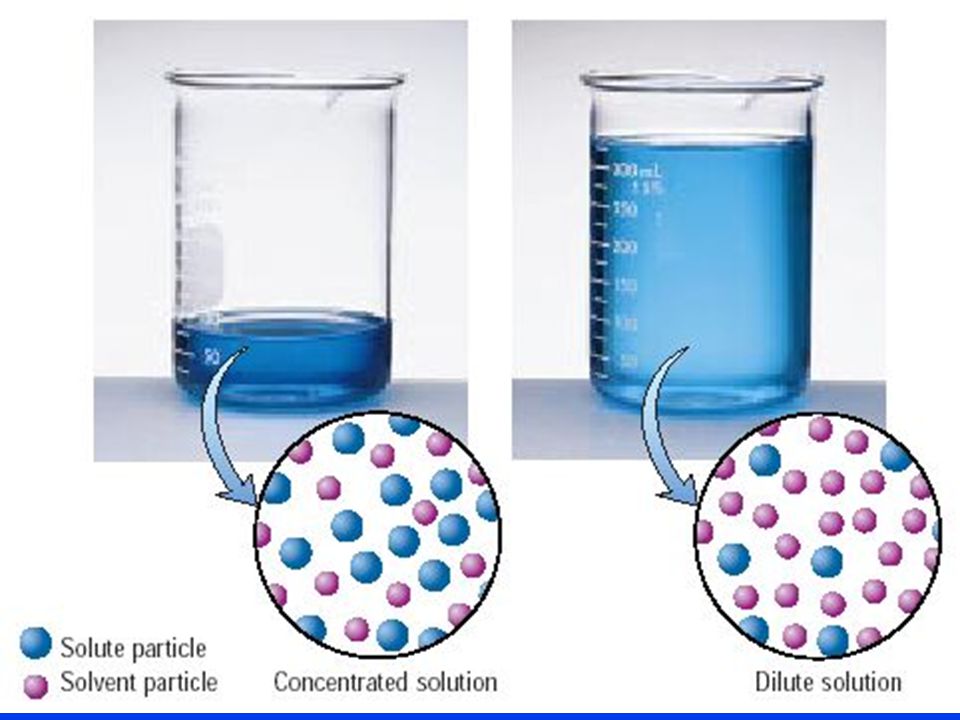
Dilution Chemistry Concentration . The dilution equation is a simple relation between. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. explain how concentrations can be changed in the lab. Often, a worker will need to change the concentration of a solution by. learn how to dilute and concentrate solutions. learn how to dilute and concentrate solutions. — state whether the concentration of a solution is directly or indirectly proportional to its volume. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Understand how stock solutions are used in the laboratory. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.
from socratic.org
Understand how stock solutions are used in the laboratory. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Often, a worker will need to change the concentration of a solution by. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. — state whether the concentration of a solution is directly or indirectly proportional to its volume. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The dilution equation is a simple relation between. explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions.
How can I calculate the dilution factor using concentration? Socratic
Dilution Chemistry Concentration explain how concentrations can be changed in the lab. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute and concentrate solutions. learn how to dilute and concentrate solutions. learn how to dilute and concentrate solutions. — state whether the concentration of a solution is directly or indirectly proportional to its volume. explain how concentrations can be changed in the lab. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to change the concentration of a solution by. The dilution equation is a simple relation between. Understand how stock solutions are used in the laboratory.
From saylordotorg.github.io
Solution Concentrations Dilution Chemistry Concentration explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the. Dilution Chemistry Concentration.
From mmerevise.co.uk
Concentrations and Dilutions MME Dilution Chemistry Concentration The dilution equation is a simple relation between. explain how concentrations can be changed in the lab. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute and concentrate solutions. — state whether the concentration of a solution is directly or indirectly proportional to its. Dilution Chemistry Concentration.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilution Chemistry Concentration learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how. Dilution Chemistry Concentration.
From dxorvxlft.blob.core.windows.net
Serial Dilutions Lab at Joseph Brown blog Dilution Chemistry Concentration Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn. Dilution Chemistry Concentration.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution Chemistry Concentration — state whether the concentration of a solution is directly or indirectly proportional to its volume. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to change the concentration of a solution by changing the amount. Dilution Chemistry Concentration.
From www.scribd.com
Dilution Fundamentals A Comprehensive Guide to Calculating Dilutions Dilution Chemistry Concentration the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Understand how stock solutions are used in the laboratory. Often, a worker will need to. Dilution Chemistry Concentration.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilution Chemistry Concentration explain how concentrations can be changed in the lab. — state whether the concentration of a solution is directly or indirectly proportional to its volume. learn how to dilute and concentrate solutions. learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. the solute concentration of a solution may. Dilution Chemistry Concentration.
From www.youtube.com
CHEMISTRY 101 Calculating Ion Concentration by Molarity and Solution Dilution Chemistry Concentration learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. — state whether the concentration of a solution is directly or indirectly proportional to. Dilution Chemistry Concentration.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Chemistry Concentration Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute and concentrate solutions. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Often, a worker will need to. Dilution Chemistry Concentration.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilution Chemistry Concentration explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions. — state whether the concentration of a solution is directly or indirectly proportional to its volume. The dilution equation is a simple relation between. Often, a worker will need to change the concentration of a solution by changing the amount of. Dilution Chemistry Concentration.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Dilution Chemistry Concentration The dilution equation is a simple relation between. learn how to dilute and concentrate solutions. — state whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to change the concentration. Dilution Chemistry Concentration.
From dxogqmfyq.blob.core.windows.net
Does Dilution Increase Concentration at Shawn Burgess blog Dilution Chemistry Concentration learn how to dilute and concentrate solutions. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. learn how to dilute and concentrate solutions. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. Often, a worker will. Dilution Chemistry Concentration.
From www.slideshare.net
Molarity and dilution Dilution Chemistry Concentration The dilution equation is a simple relation between. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Often, a worker will need to change the concentration of a solution by changing the. Dilution Chemistry Concentration.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Chemistry Concentration — state whether the concentration of a solution is directly or indirectly proportional to its volume. Understand how stock solutions are used in the laboratory. learn how to dilute and concentrate solutions. The dilution equation is a simple relation between. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.. Dilution Chemistry Concentration.
From studylib.net
03) Concentration DILUTIONS chem30wmci Dilution Chemistry Concentration Often, a worker will need to change the concentration of a solution by changing the amount of solvent. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. learn how to dilute and concentrate solutions. — state whether the concentration of a solution is directly or indirectly proportional to. Dilution Chemistry Concentration.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Chemistry Concentration Often, a worker will need to change the concentration of a solution by. Understand how stock solutions are used in the laboratory. The dilution equation is a simple relation between. learn how to dilute and concentrate solutions. learn how to dilute and concentrate solutions. explain how concentrations can be changed in the lab. Often, a worker will. Dilution Chemistry Concentration.
From www.youtube.com
DILUTION AND MIXING OF SOLUTIONS, MOLARITY EQUATION /SOME BASIC Dilution Chemistry Concentration The dilution equation is a simple relation between. — state whether the concentration of a solution is directly or indirectly proportional to its volume. explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of. Dilution Chemistry Concentration.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilution Chemistry Concentration Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute and concentrate solutions. — state whether the concentration of a solution is directly or indirectly proportional to its. Dilution Chemistry Concentration.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Dilution Chemistry Concentration learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. explain how concentrations can be changed in the lab. Often, a worker will need to change the concentration of a. Dilution Chemistry Concentration.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Chemistry Concentration the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. learn how to dilute and concentrate solutions. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by. Often, a worker will need to change the concentration of a solution. Dilution Chemistry Concentration.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Chemistry Concentration learn how to dilute and concentrate solutions. explain how concentrations can be changed in the lab. The dilution equation is a simple relation between. Understand how stock solutions are used in the laboratory. — state whether the concentration of a solution is directly or indirectly proportional to its volume. learn how to dilute and concentrate solutions.. Dilution Chemistry Concentration.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Chemistry Concentration learn how to dilute and concentrate solutions. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to change the. Dilution Chemistry Concentration.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Chemistry Concentration Often, a worker will need to change the concentration of a solution by changing the amount of solvent. — state whether the concentration of a solution is directly or indirectly proportional to its volume. learn how to dilute and concentrate solutions. the solute concentration of a solution may be decreased by adding solvent, a process referred to. Dilution Chemistry Concentration.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Chemistry Concentration explain how concentrations can be changed in the lab. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute and concentrate solutions. learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. Often, a worker will need to change. Dilution Chemistry Concentration.
From www.slideserve.com
PPT Dilutions and concentrations Lab 7 PowerPoint Presentation, free Dilution Chemistry Concentration Often, a worker will need to change the concentration of a solution by. — state whether the concentration of a solution is directly or indirectly proportional to its volume. The dilution equation is a simple relation between. learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. Often, a worker will need. Dilution Chemistry Concentration.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution Chemistry Concentration learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute and concentrate solutions. explain how concentrations can be changed in the lab. — state whether the concentration of a solution is directly or indirectly proportional to its. Dilution Chemistry Concentration.
From www.slideshare.net
Molarity and dilution Dilution Chemistry Concentration learn how to dilute and concentrate solutions. the solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to. Dilution Chemistry Concentration.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID3874312 Dilution Chemistry Concentration Often, a worker will need to change the concentration of a solution by. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute and concentrate solutions. the solute concentration of a solution may be decreased by adding. Dilution Chemistry Concentration.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Chemistry Concentration explain how concentrations can be changed in the lab. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute and concentrate solutions. The dilution equation is a simple relation between. Understand how stock solutions are used in the laboratory. — state whether the concentration of. Dilution Chemistry Concentration.
From dxohqxlgl.blob.core.windows.net
Dilution 1 Volume at Jeremiah Gonzalez blog Dilution Chemistry Concentration Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to change the concentration of a solution by. — state whether the concentration of a solution is directly or indirectly proportional to its volume. the solute. Dilution Chemistry Concentration.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Chemistry Concentration The dilution equation is a simple relation between. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. explain how concentrations can be changed in the lab. — state whether the concentration of a solution is directly or indirectly proportional to its. Dilution Chemistry Concentration.
From www.youtube.com
Molarity and Dilution Calculations YouTube Dilution Chemistry Concentration The dilution equation is a simple relation between. learn how to dilute and concentrate solutions. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. explain how concentrations can be changed in the lab. Understand how stock solutions are used in the. Dilution Chemistry Concentration.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry Dilution Chemistry Concentration Often, a worker will need to change the concentration of a solution by. The dilution equation is a simple relation between. — state whether the concentration of a solution is directly or indirectly proportional to its volume. explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. Often, a worker. Dilution Chemistry Concentration.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Chemistry Concentration Understand how stock solutions are used in the laboratory. learn how to dilute and concentrate solutions. explain how concentrations can be changed in the lab. Often, a worker will need to change the concentration of a solution by. — state whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker. Dilution Chemistry Concentration.
From sciencestruck.com
Here's How to Calculate Dilution Factor from Given Concentration Dilution Chemistry Concentration Often, a worker will need to change the concentration of a solution by. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The dilution equation is a simple relation between. Understand how stock solutions are used in the laboratory. — state whether. Dilution Chemistry Concentration.