Catalyst Organic Chemistry Mechanism . These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. The advantages of organocatalysts include their. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant.
from www.masterorganicchemistry.com
An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. The advantages of organocatalysts include their. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant.
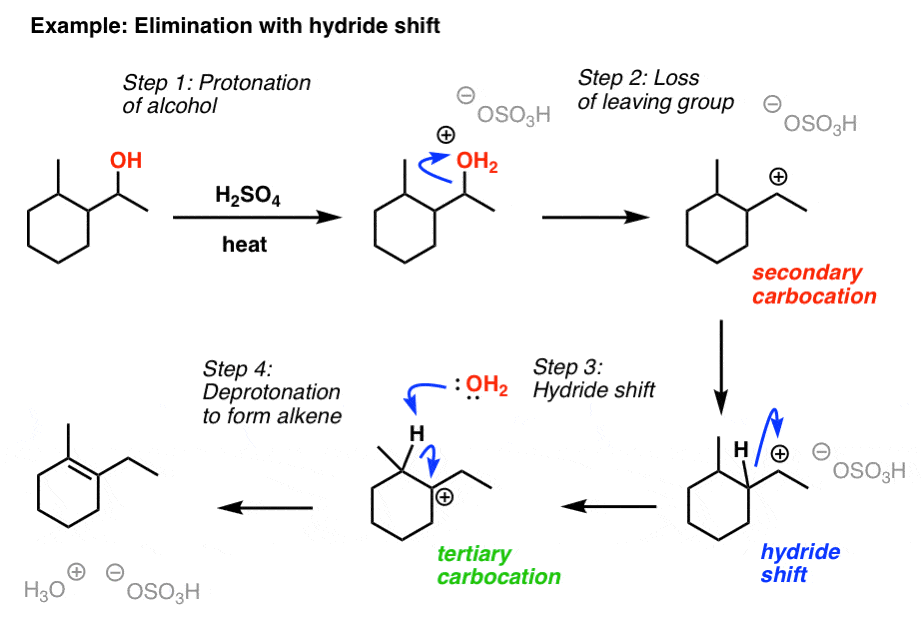
Elimination Reactions of Alcohols Master Organic Chemistry
Catalyst Organic Chemistry Mechanism An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. The advantages of organocatalysts include their. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant.
From chemistnotes.com
Phase transfer catalyst, Easy Mechanism Chemistry Notes Catalyst Organic Chemistry Mechanism An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. The advantages of organocatalysts include their. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. A catalyst increases the rate of a reaction, but. Catalyst Organic Chemistry Mechanism.
From www.pinterest.com
The Mechanism of BaseCatalyzed Hydrolysis of Nitriles Organic Catalyst Organic Chemistry Mechanism The advantages of organocatalysts include their. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. An overview of the basic photophysics and electron transfer theory is presented. Catalyst Organic Chemistry Mechanism.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Organic Chemistry Mechanism A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. Organocatalysis uses small organic molecules predominantly composed of c, h,. Catalyst Organic Chemistry Mechanism.
From courses.lumenlearning.com
19.4. Reduction of alkenes and alkynes Organic Chemistry II Catalyst Organic Chemistry Mechanism An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. A catalyst increases the rate of a reaction, but does not get consumed in the. Catalyst Organic Chemistry Mechanism.
From www.slideserve.com
PPT Surfactants as Catalysts for Organic Reactions in Water Catalyst Organic Chemistry Mechanism The advantages of organocatalysts include their. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s. Catalyst Organic Chemistry Mechanism.
From www.masterorganicchemistry.com
Alcohols To Ethers via Acid Catalysis Master Organic Chemistry Catalyst Organic Chemistry Mechanism An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. These materials bridge the gap between organometallic and nanoparticle catalysis. Catalyst Organic Chemistry Mechanism.
From www.organicchemistrytutor.com
FriedelCrafts Alkylation and Acylation Reaction — Organic Chemistry Tutor Catalyst Organic Chemistry Mechanism A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive. Catalyst Organic Chemistry Mechanism.
From www.vedantu.com
Birch Reduction and Lindlar Catalyst Important Concepts and Tips for JEE Catalyst Organic Chemistry Mechanism Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes.. Catalyst Organic Chemistry Mechanism.
From www.youtube.com
Phase Transfer Catalyst (PTC) Chemistry by Dr. Tanmoy Biswas Catalyst Organic Chemistry Mechanism An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting. Catalyst Organic Chemistry Mechanism.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Catalyst Organic Chemistry Mechanism A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. These materials bridge the gap between organometallic and nanoparticle catalysis. Catalyst Organic Chemistry Mechanism.
From www.youtube.com
Acidcatalyzed ester hydrolysis Organic chemistry Khan Academy Catalyst Organic Chemistry Mechanism These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. The advantages of organocatalysts include their. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. A catalyst increases the rate of a reaction, but. Catalyst Organic Chemistry Mechanism.
From testbook.com
Wilkinson’s Catalyst Learn Definition, Mechanism & Preparation Catalyst Organic Chemistry Mechanism These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. The advantages of organocatalysts include their. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. An overview of the basic photophysics and electron transfer theory is presented in. Catalyst Organic Chemistry Mechanism.
From www.masterorganicchemistry.com
Elimination Reactions of Alcohols Master Organic Chemistry Catalyst Organic Chemistry Mechanism Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant.. Catalyst Organic Chemistry Mechanism.
From leah4sci.com
Organic Chemistry Mechanisms Tutorial Video Series by Leah4Sci Catalyst Organic Chemistry Mechanism Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant.. Catalyst Organic Chemistry Mechanism.
From www.researchgate.net
Proposed mechanisms for the DBU catalyzed ureasynthesis reaction from Catalyst Organic Chemistry Mechanism A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide. Catalyst Organic Chemistry Mechanism.
From www.masterorganicchemistry.com
Olefin Metathesis Master Organic Chemistry Catalyst Organic Chemistry Mechanism The advantages of organocatalysts include their. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. An overview of the basic photophysics and electron transfer theory is presented. Catalyst Organic Chemistry Mechanism.
From www.pinterest.com.mx
Synthesis (4) Alkene Reaction Map, Including Alkyl Halide Reactions Catalyst Organic Chemistry Mechanism Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. The advantages of organocatalysts include their. These materials bridge the gap between organometallic and nanoparticle catalysis and are. Catalyst Organic Chemistry Mechanism.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst Organic Chemistry Mechanism The advantages of organocatalysts include their. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and. Catalyst Organic Chemistry Mechanism.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID4409461 Catalyst Organic Chemistry Mechanism These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. The advantages of organocatalysts include their. Organocatalysis uses small organic molecules predominantly composed of c,. Catalyst Organic Chemistry Mechanism.
From www.chemistrysteps.com
Amide Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Catalyst Organic Chemistry Mechanism A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive. Catalyst Organic Chemistry Mechanism.
From pubs.acs.org
Photoredox Catalysis in Organic Chemistry The Journal of Organic Catalyst Organic Chemistry Mechanism An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. The advantages of organocatalysts include their. These materials bridge the gap between organometallic and. Catalyst Organic Chemistry Mechanism.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Catalyst Organic Chemistry Mechanism The advantages of organocatalysts include their. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s. Catalyst Organic Chemistry Mechanism.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Organic Chemistry Mechanism These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions.. Catalyst Organic Chemistry Mechanism.
From www.mdpi.com
Catalysts Free FullText CopperBased Frameworks Catalyst Organic Chemistry Mechanism Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. The advantages of organocatalysts include their. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. An overview of the basic photophysics and electron transfer theory is presented. Catalyst Organic Chemistry Mechanism.
From www.masterorganicchemistry.com
Acid Catalysis of Organic Reactions Why It Works Catalyst Organic Chemistry Mechanism These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p. Catalyst Organic Chemistry Mechanism.
From www.youtube.com
01.10 Base Catalysis and Summary YouTube Catalyst Organic Chemistry Mechanism These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant.. Catalyst Organic Chemistry Mechanism.
From www.masterorganicchemistry.com
Elimination Reactions of Alcohols Master Organic Chemistry Catalyst Organic Chemistry Mechanism The advantages of organocatalysts include their. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. A catalyst increases the rate of a reaction, but. Catalyst Organic Chemistry Mechanism.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Organic Chemistry Mechanism Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. The advantages of organocatalysts include their. An overview of the basic photophysics and electron transfer theory is presented. Catalyst Organic Chemistry Mechanism.
From www.masterorganicchemistry.com
Palladium on Carbon (Pd/C) for Catalytic Hydrogenation of Alkenes Catalyst Organic Chemistry Mechanism The advantages of organocatalysts include their. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. A catalyst increases the rate of a reaction, but. Catalyst Organic Chemistry Mechanism.
From www.masterorganicchemistry.com
Transesterification Master Organic Chemistry Catalyst Organic Chemistry Mechanism These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions.. Catalyst Organic Chemistry Mechanism.
From www.mdpi.com
Catalysts Free FullText Photocatalyzed Oxygenation Reactions with Catalyst Organic Chemistry Mechanism A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide. Catalyst Organic Chemistry Mechanism.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Organic Chemistry Mechanism These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. An overview of the basic photophysics and electron transfer theory is presented in order to provide a comprehensive guide for employing this class of catalysts in photoredox. The advantages of organocatalysts include their. A catalyst increases the rate of a reaction, but. Catalyst Organic Chemistry Mechanism.
From www.masterorganicchemistry.com
Olefin Metathesis Master Organic Chemistry Catalyst Organic Chemistry Mechanism These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. The advantages of organocatalysts include their. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. An overview of the basic photophysics and electron transfer theory is presented in. Catalyst Organic Chemistry Mechanism.
From www.masterorganicchemistry.com
Summary Three Key Families Of Alkene Reaction Mechanisms Catalyst Organic Chemistry Mechanism A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes.. Catalyst Organic Chemistry Mechanism.
From www.researchgate.net
Scheme 22. General catalytic cycle of a Pd(0)/Pd(II) intermolecular Catalyst Organic Chemistry Mechanism The advantages of organocatalysts include their. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. Organocatalysis uses small organic molecules predominantly composed of c, h, o, n, s and p to accelerate chemical reactions. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and. Catalyst Organic Chemistry Mechanism.