Can Salt Dissolve Into Ethanol . This is done by following these steps: If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. The salt, however, did not dissolve as easily in the rubbing alcohol in. Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. This method works because the. A salt solution (salt water) can be distilled into pure water which we can drink.
from www.slideserve.com
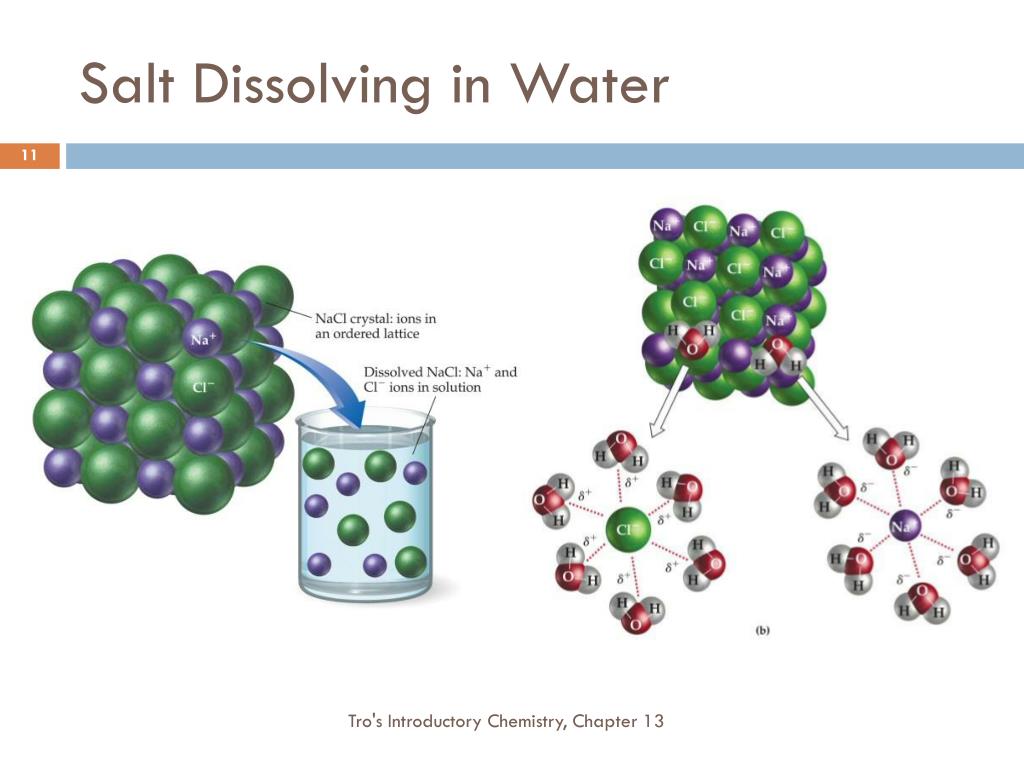
This method works because the. The salt, however, did not dissolve as easily in the rubbing alcohol in. This is done by following these steps: For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. A salt solution (salt water) can be distilled into pure water which we can drink. Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide.
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID
Can Salt Dissolve Into Ethanol For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. The salt, however, did not dissolve as easily in the rubbing alcohol in. A salt solution (salt water) can be distilled into pure water which we can drink. This is done by following these steps: Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. This method works because the. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless.
From www.gbu-presnenskij.ru
Solubility Of Organic Compounds Chemistry Steps, 53 OFF Can Salt Dissolve Into Ethanol Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. This method works because the. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. Remember that this occurs because the ionic salt molecules. Can Salt Dissolve Into Ethanol.
From studyzonequaaludes.z13.web.core.windows.net
Separating Water And Salt Can Salt Dissolve Into Ethanol Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. The salt, however, did not dissolve as easily in the rubbing alcohol in. A salt solution (salt water) can be distilled into pure water which we can. Can Salt Dissolve Into Ethanol.
From www.teachoo.com
[Carbon Class 10] Ethanol Physical and Chemical Properties, Uses Can Salt Dissolve Into Ethanol This is done by following these steps: If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. Salt can dissolve in a wide range of alcohols, including ethanol (the type of. Can Salt Dissolve Into Ethanol.
From www.youtube.com
DISTILLATION OF WATER AND ETHANOL YouTube Can Salt Dissolve Into Ethanol Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. This method works because the. A salt solution (salt water) can be distilled into pure water which we can drink. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. If. Can Salt Dissolve Into Ethanol.
From courses.lumenlearning.com
The Dissolution Process Chemistry Can Salt Dissolve Into Ethanol Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. The salt, however, did not dissolve as easily in the rubbing alcohol in. This is done by following these steps: This method. Can Salt Dissolve Into Ethanol.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Can Salt Dissolve Into Ethanol The salt, however, did not dissolve as easily in the rubbing alcohol in. This is done by following these steps: If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found. Can Salt Dissolve Into Ethanol.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Can Salt Dissolve Into Ethanol For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. This method works because the.. Can Salt Dissolve Into Ethanol.
From www.youtube.com
Separation of Ethanol and Water with Salt YouTube Can Salt Dissolve Into Ethanol This method works because the. This is done by following these steps: Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. For example,. Can Salt Dissolve Into Ethanol.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Can Salt Dissolve Into Ethanol Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. The salt, however, did not dissolve as easily in the rubbing alcohol in. Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. This is done by following these steps: This method. Can Salt Dissolve Into Ethanol.
From www.youtube.com
Is C2H5OH (Ethanol) Soluble or Insoluble in Water? YouTube Can Salt Dissolve Into Ethanol For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. This is done by following these steps: The salt, however, did not dissolve as easily in the rubbing alcohol. Can Salt Dissolve Into Ethanol.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Can Salt Dissolve Into Ethanol Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. A salt solution (salt water) can be distilled into pure water which we can drink. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. This. Can Salt Dissolve Into Ethanol.
From www.youtube.com
Solubility of salts and preparation of soluble salts YouTube Can Salt Dissolve Into Ethanol Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional. Can Salt Dissolve Into Ethanol.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? Can Salt Dissolve Into Ethanol The salt, however, did not dissolve as easily in the rubbing alcohol in. A salt solution (salt water) can be distilled into pure water which we can drink. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. For example, liquid ethanol can be separated from. Can Salt Dissolve Into Ethanol.
From www.labkafe.com
Solubility of Salts ‒ Why Common Salts are So Soluble in Water Labkafe Can Salt Dissolve Into Ethanol If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. The salt, however, did not dissolve as easily in the rubbing alcohol in. If a small. Can Salt Dissolve Into Ethanol.
From pubs.acs.org
Saltingout and Competitive Adsorption of Ethanol into Lipid Bilayer Can Salt Dissolve Into Ethanol If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. Remember that this occurs because the ionic salt molecules easily bond to the polar. Can Salt Dissolve Into Ethanol.
From www.slideserve.com
PPT Chapter 7 Chemical Reactions PowerPoint Presentation, free Can Salt Dissolve Into Ethanol This is done by following these steps: A salt solution (salt water) can be distilled into pure water which we can drink. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. For example, liquid ethanol can be separated from a mixture of ethanol and water. Can Salt Dissolve Into Ethanol.
From www.acs.org
Lesson 5.3 Why Does Water Dissolve Salt? American Chemical Society Can Salt Dissolve Into Ethanol If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. A salt solution (salt water) can be distilled into pure water which we can. Can Salt Dissolve Into Ethanol.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Can Salt Dissolve Into Ethanol For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid. Can Salt Dissolve Into Ethanol.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures Can Salt Dissolve Into Ethanol A salt solution (salt water) can be distilled into pure water which we can drink. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. This is done by following these steps: The salt, however, did not dissolve as easily in the rubbing alcohol in. Remember. Can Salt Dissolve Into Ethanol.
From www.youtube.com
Mixing ethanol and water YouTube Can Salt Dissolve Into Ethanol A salt solution (salt water) can be distilled into pure water which we can drink. The salt, however, did not dissolve as easily in the rubbing alcohol in. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. Remember that this occurs because the ionic salt. Can Salt Dissolve Into Ethanol.
From www.youtube.com
Reaction of Sodium with Ethanol 002 YouTube Can Salt Dissolve Into Ethanol Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. This is done by following these steps: A salt solution (salt water) can be distilled into pure water which we can drink.. Can Salt Dissolve Into Ethanol.
From slideplayer.com
Unit 2 Properties of Matter ppt download Can Salt Dissolve Into Ethanol If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. The salt, however, did not dissolve as easily in the rubbing alcohol in. This method works because the. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the. Can Salt Dissolve Into Ethanol.
From www.bbc.co.uk
Distillation BBC Bitesize Can Salt Dissolve Into Ethanol Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. A salt solution (salt water) can be distilled into pure water which we can drink. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. Salt can dissolve in a wide range. Can Salt Dissolve Into Ethanol.
From www.chemicals.co.uk
How is Ethanol Converted into Ethanoic Acid? Can Salt Dissolve Into Ethanol Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. The salt, however, did not dissolve as easily in the rubbing alcohol in. A salt solution (salt water) can be distilled into pure water which we can drink. If a small piece of sodium is dropped into ethanol,. Can Salt Dissolve Into Ethanol.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6540865 Can Salt Dissolve Into Ethanol Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. A salt solution (salt water) can be distilled into pure water which we can drink. The salt, however, did not dissolve as easily in the rubbing alcohol in. This method works because the. For example, liquid ethanol can be separated from a mixture of. Can Salt Dissolve Into Ethanol.
From www.slideserve.com
PPT Polar water molecules interacting with positive and negative ions Can Salt Dissolve Into Ethanol This method works because the. This is done by following these steps: Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. The salt, however, did not dissolve as easily in the rubbing alcohol in. A salt solution (salt water) can be distilled into pure water which we. Can Salt Dissolve Into Ethanol.
From www.gauthmath.com
Solved Fats will dissolve in ethanol. Ethanol is an example of a Can Salt Dissolve Into Ethanol If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. For example, liquid ethanol can be separated from a mixture of ethanol and water. Can Salt Dissolve Into Ethanol.
From passmyexams.co.uk
Oxidation of Ethanol Easy exam revision notes for GSCE Chemistry Can Salt Dissolve Into Ethanol For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the. Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. A salt solution (salt water) can be distilled into pure water which we can drink. If. Can Salt Dissolve Into Ethanol.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Can Salt Dissolve Into Ethanol For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the. A salt solution (salt water) can be distilled into pure water which we can drink. This is done by following these steps: Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found. Can Salt Dissolve Into Ethanol.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Can Salt Dissolve Into Ethanol Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves. Can Salt Dissolve Into Ethanol.
From www.shalom-education.com
Ionic Compounds Edexcel GCSE Chemistry Revision Can Salt Dissolve Into Ethanol This is done by following these steps: A salt solution (salt water) can be distilled into pure water which we can drink. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless. This method works because the. The salt, however, did not dissolve as easily in. Can Salt Dissolve Into Ethanol.
From educatorpages.com
Chemistry Can Salt Dissolve Into Ethanol Salt can dissolve in a wide range of alcohols, including ethanol (the type of alcohol found in alcoholic beverages), methanol, and isopropyl. The salt, however, did not dissolve as easily in the rubbing alcohol in. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. A salt solution (salt water) can be distilled. Can Salt Dissolve Into Ethanol.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Can Salt Dissolve Into Ethanol If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. This method works because the. Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles. Can Salt Dissolve Into Ethanol.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Can Salt Dissolve Into Ethanol This method works because the. If a small piece of sodium is dropped into ethanol, bubbles of hydrogen gas are produced and the liquid contains sodium ethoxide. The salt, however, did not dissolve as easily in the rubbing alcohol in. A salt solution (salt water) can be distilled into pure water which we can drink. This is done by following. Can Salt Dissolve Into Ethanol.
From yesdirt.com
Does Salt Dissolve In Alcohol? (ANSWERED) Yes Dirt Can Salt Dissolve Into Ethanol Remember that this occurs because the ionic salt molecules easily bond to the polar water molecules. A salt solution (salt water) can be distilled into pure water which we can drink. This method works because the. If a small piece of sodium is dropped into ethanol, it reacts steadily to give off bubbles of hydrogen gas and leaves a colorless.. Can Salt Dissolve Into Ethanol.