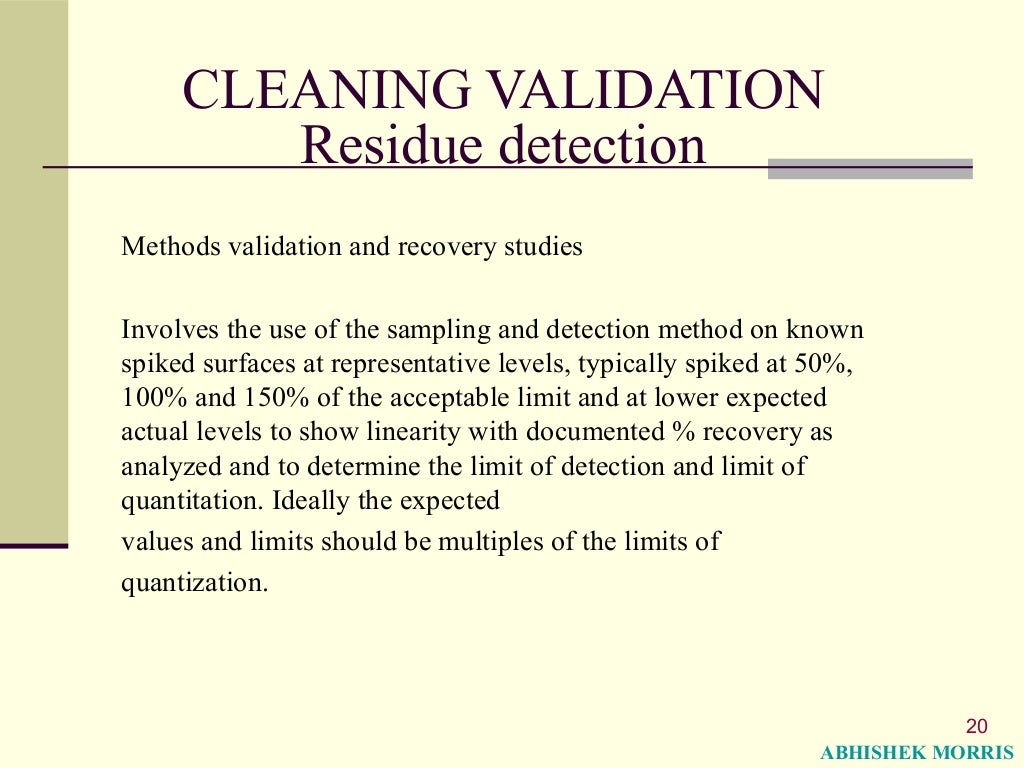
Cleaning Validation Meaning . 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. This guide is designed to.
from www.slideshare.net
12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. This guide is designed to. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch.
Basic Concepts of Cleaning validation
Cleaning Validation Meaning Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. This guide is designed to. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover.
From www.slideshare.net
Cleaning validation strategy & regulations Cleaning Validation Meaning These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. This guide is designed to. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. 12.7. Cleaning Validation Meaning.
From safetyculture.com
Cleaning Validation Protocol & Guidelines SafetyCulture Cleaning Validation Meaning This guide is designed to. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Cleaning validation confirms that any leftover materials are removed to make sure the next. Cleaning Validation Meaning.
From www.slideserve.com
PPT Cleaning Validation PowerPoint Presentation, free download ID Cleaning Validation Meaning Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Cleaning validation in the pharmaceutical industry is a regulatory. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning validation a complete know how Cleaning Validation Meaning Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. This guide is designed to. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or.. Cleaning Validation Meaning.
From www.slideshare.net
Basic Concepts of Cleaning validation Cleaning Validation Meaning 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. This guide is designed to. Companies must demonstrate. Cleaning Validation Meaning.
From www.factssa.com
Infographic The difference between Allergen Cleaning Validation and Cleaning Validation Meaning 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. This guide is designed to. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning validation a complete know how Cleaning Validation Meaning Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and. Cleaning Validation Meaning.
From www.youtube.com
Requirement and Reasons of Cleaning Validation! Basics of cleaning Cleaning Validation Meaning These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Learn about the basics of cleaning. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning validation a complete know how Cleaning Validation Meaning Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation.. Cleaning Validation Meaning.
From dokumen.tips
(PDF) Cleaning Validation Presentation.ppt ISPE Boston 3 Definition Cleaning Validation Meaning Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left. Cleaning Validation Meaning.
From www.slideshare.net
Basic Concepts of Cleaning validation Cleaning Validation Meaning Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Companies must. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning validation Cleaning Validation Meaning These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. This guide is designed to. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. Cleaning. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning validation a complete know how Cleaning Validation Meaning Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. This guide is designed to. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece. Cleaning Validation Meaning.
From slideplayer.com
VALIDATION OF PHARMACEUTICAL PROCESSES ppt download Cleaning Validation Meaning This guide is designed to. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. These agency documents clearly establish the expectation that cleaning procedures. Cleaning Validation Meaning.
From pharmaceuticalsindex.com
Importance of Cleaning Validation Pharmaceuticals Index Cleaning Validation Meaning This guide is designed to. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. Cleaning validation in the pharmaceutical industry is a regulatory. Cleaning Validation Meaning.
From medvacon.com
Cleaning Validation — Medvacon Life Sciences LLC Cleaning Validation Meaning 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece. Cleaning Validation Meaning.
From www.presentationeze.com
Cleaning Validation.PresentationEZE Cleaning Validation Meaning 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as. Cleaning Validation Meaning.
From www.slideserve.com
PPT Validation of Cleaning Processes PowerPoint Presentation, free Cleaning Validation Meaning Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Learn about the basics of cleaning validation, fda. Cleaning Validation Meaning.
From www.slideshare.net
Basic Concepts of Cleaning validation Cleaning Validation Meaning These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Cleaning validation in the pharmaceutical industry is a. Cleaning Validation Meaning.
From www.slideserve.com
PPT 3. Validation (and Qualification) PowerPoint Presentation, free Cleaning Validation Meaning Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning validation a complete know how Cleaning Validation Meaning 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. This guide is designed to. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured. Cleaning Validation Meaning.
From www.leucine.io
Cleaning Validation Guidelines A Complete List 2022 Cleaning Validation Meaning Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,.. Cleaning Validation Meaning.
From slideplayer.com
CLEANING VALIDATION BASIC CONCEPTS. ppt download Cleaning Validation Meaning This guide is designed to. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning validation Cleaning Validation Meaning 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Cleaning. Cleaning Validation Meaning.
From www.researchgate.net
(PDF) PROCEDURE FOR CLEANING VALIDATION Cleaning Validation Meaning Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Cleaning validation. Cleaning Validation Meaning.
From www.researchgate.net
(PDF) CLEANING VALIDATION Cleaning Validation Meaning Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. 12.7 cleaning validation • validation of. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning validation Cleaning Validation Meaning This guide is designed to. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning Validation "Part 1" Cleaning Validation Meaning Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. This guide is designed to. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Cleaning. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning validation Cleaning Validation Meaning 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as. Cleaning Validation Meaning.
From www.youtube.com
10 Important Terms you must know in CLEANING VALIDATION YouTube Cleaning Validation Meaning Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. This guide is designed to. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. Companies must demonstrate during validation that the cleaning procedure routinely. Cleaning Validation Meaning.
From www.slideshare.net
Fundamental of cleaning validation Cleaning Validation Meaning This guide is designed to. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how. Cleaning Validation Meaning.
From www.slideshare.net
Basic Concepts of Cleaning validation Cleaning Validation Meaning Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation.. Cleaning Validation Meaning.
From www.slideshare.net
Basic Concepts of Cleaning validation Cleaning Validation Meaning Cleaning validation in the pharmaceutical industry is a regulatory requirement to ensure equipment cleaning procedures effectively remove all residues, such as actives,. Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. This guide is designed to. 12.7 cleaning validation • validation of cleaning. Cleaning Validation Meaning.
From www.youtube.com
Cleaning Validation in 10 Steps Cleaning Validation in Cleaning Validation Meaning This guide is designed to. Learn about the basics of cleaning validation, fda guidelines and protocol development guide questions, and how a cleaning validation. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. These agency documents clearly establish the expectation that cleaning procedures (processes) be validated. Cleaning validation confirms. Cleaning Validation Meaning.
From www.slideshare.net
Cleaning validation a complete know how Cleaning Validation Meaning Cleaning validation confirms that any leftover materials are removed to make sure the next product manufactured is not affected by anything left from the previous batch. Companies must demonstrate during validation that the cleaning procedure routinely employed for a piece of equipment limits potential carryover. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71). Cleaning Validation Meaning.