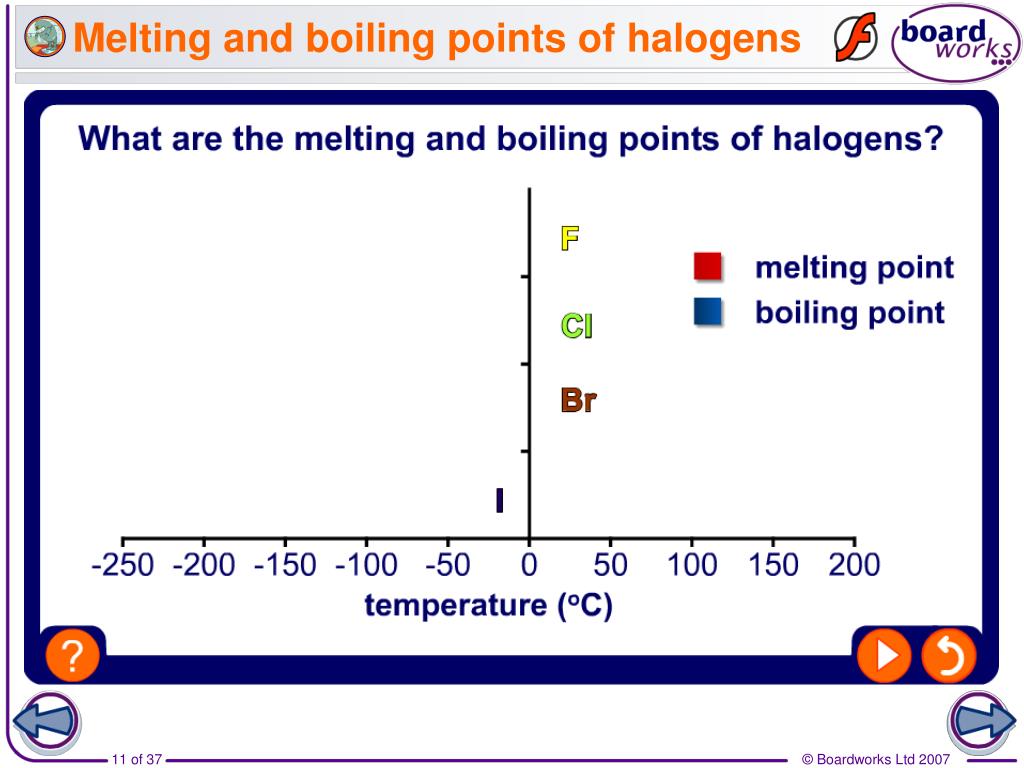
Boiling Point Properties Of Halogens . Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Boiling point increases down the group. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. The halogens exist as simple. This is because it has the weakest van der waals. Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. Physical properties of the halogens. Predict the melting and boiling points of astatine, and its state at room temperature. Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. Each molecule contains two halogen. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the. We can explain this trend by looking at.
from www.slideserve.com
Physical properties of the halogens. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the. Predict the melting and boiling points of astatine, and its state at room temperature. Each molecule contains two halogen. Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. We can explain this trend by looking at. Boiling point increases down the group. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond.
PPT Group 7 the halogens PowerPoint Presentation, free download
Boiling Point Properties Of Halogens Physical properties of the halogens. Predict the melting and boiling points of astatine, and its state at room temperature. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Boiling point increases down the group. Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the. Physical properties of the halogens. The halogens exist as simple. We can explain this trend by looking at. This is because it has the weakest van der waals. Each molecule contains two halogen.
From courses.lumenlearning.com
Intermolecular Forces Chemistry Atoms First Boiling Point Properties Of Halogens Boiling point increases down the group. We can explain this trend by looking at. Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond.. Boiling Point Properties Of Halogens.
From www.researchgate.net
Boiling points of halogens of selected MRAM materials. Download Boiling Point Properties Of Halogens Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. This is because it has the weakest van der waals. The halogens exist as simple. Each molecule contains two halogen. We can explain this trend by looking at. Predict the melting and boiling points of astatine, and its state at room temperature. It. Boiling Point Properties Of Halogens.
From slideplayer.com
Melting and Boiling points? ppt download Boiling Point Properties Of Halogens The halogens exist as simple. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Physical properties of the halogens. This is because it has the weakest van der waals. We can explain this trend by looking at. Astatine should have a melting point. Boiling Point Properties Of Halogens.
From www.gauthmath.com
Solved The boiling points of diatomic halogens are compared in the Boiling Point Properties Of Halogens Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. The halogens exist as simple. This is because it has the. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT INTERMOLECULAR FORCES OF ATTRACTION PowerPoint Presentation, free Boiling Point Properties Of Halogens It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6702617 Boiling Point Properties Of Halogens The halogens exist as simple. Physical properties of the halogens. Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. It can be seen. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Boiling Point Properties Of Halogens It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Each molecule contains two halogen. Boiling point increases down the group. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond.. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Boiling Point Properties Of Halogens Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. Boiling point increases down the group. We can explain this trend by looking at. The halogens exist as simple. There is. Boiling Point Properties Of Halogens.
From www.researchgate.net
Some properties of the Halogens Download Table Boiling Point Properties Of Halogens Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. There is a regular increase in many of the properties of. Boiling Point Properties Of Halogens.
From chempedia.info
Boiling point of halogens Big Chemical Encyclopedia Boiling Point Properties Of Halogens This is because it has the weakest van der waals. Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. Predict the melting and boiling points of astatine, and its state at room temperature. The halogens exist as simple. Astatine should have a melting point of about 300°c and a boiling point of. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Boiling Point Properties Of Halogens Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. We can explain this trend by looking at. Boiling point increases down the group. Each molecule contains two halogen. Sections below cover the trends in atomic radius,. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Chapter 22 Chemistry of the Nonmetals PowerPoint Presentation Boiling Point Properties Of Halogens Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. This is because it has the weakest van der waals. Boiling point increases down the group. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT melting & boiling points PowerPoint Presentation, free download Boiling Point Properties Of Halogens We can explain this trend by looking at. Each molecule contains two halogen. Boiling point increases down the group. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the. It can be seen that there is a regular increase in many of the properties of. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Group 17 The Halogens PowerPoint Presentation, free download Boiling Point Properties Of Halogens This is because it has the weakest van der waals. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the. Each molecule contains two halogen. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion. Boiling Point Properties Of Halogens.
From periodictableguide.com
Where are the Halogens located on the Periodic table? Boiling Point Properties Of Halogens Astatine should have a melting point of about 300°c and a boiling point of about 340°c. This is because it has the weakest van der waals. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Sections below cover the trends in atomic radius,. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Boiling Point Properties Of Halogens Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Boiling point increases down the group. The halogens exist as simple. There is a regular increase in many of the properties of the halogens as we proceed. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Periodic Commonalities halogens PowerPoint Presentation, free Boiling Point Properties Of Halogens Each molecule contains two halogen. Predict the melting and boiling points of astatine, and its state at room temperature. The halogens exist as simple. Physical properties of the halogens. Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. It can be seen that there is a regular increase in many of the. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Boiling Point Properties Of Halogens Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. We can explain this trend by looking at. Boiling point increases down the group. Predict the melting and boiling points of astatine, and its state at room temperature. It can be seen that there is a regular. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID5566024 Boiling Point Properties Of Halogens Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. The halogens exist as simple. There is a regular increase in many of the properties of the halogens as we proceed. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Boiling Point Properties Of Halogens Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. Each molecule contains two halogen. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. The halogens exist as simple. There is a regular increase in many of the properties of. Boiling Point Properties Of Halogens.
From shiken.ai
Properties of Halogens Boiling Point Properties Of Halogens Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Boiling point increases down the group. Physical properties of the halogens. Each molecule contains two halogen. Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. We can explain this trend. Boiling Point Properties Of Halogens.
From www.chemistrystudent.com
Group 7 Halogens Boiling Points (ALevel) ChemistryStudent Boiling Point Properties Of Halogens Boiling point increases down the group. Each molecule contains two halogen. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. This is because it has the weakest van der waals. Melting and boiling points (increase down the group) the melting and boiling points increase down the. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Boiling Point Properties Of Halogens Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. Boiling point increases down the group. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. This is because it has the weakest van der waals. It can be seen that there is a regular increase in. Boiling Point Properties Of Halogens.
From collegedunia.com
Halogens Properties, Electronic Configuration & Characteristics Boiling Point Properties Of Halogens Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. The halogens exist as simple. Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. Each molecule contains two halogen. We can explain this trend by looking at. It can. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Boiling Point Properties Of Halogens Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of the bond. Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. We can explain this. Boiling Point Properties Of Halogens.
From shiken.ai
Properties of Halogens Boiling Point Properties Of Halogens This is because it has the weakest van der waals. Each molecule contains two halogen. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the. Predict the melting and boiling points of astatine, and its state at room temperature. We can explain this trend by. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Boiling Point Properties Of Halogens Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Physical properties of the halogens. The halogens exist as simple. We can explain this trend by looking at. Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. Sections below cover. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Boiling Point Properties Of Halogens Physical properties of the halogens. We can explain this trend by looking at. Boiling point increases down the group. Predict the melting and boiling points of astatine, and its state at room temperature. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the. Each molecule. Boiling Point Properties Of Halogens.
From slideplayer.com
Halogens. ppt download Boiling Point Properties Of Halogens Boiling point increases down the group. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. Sections below cover the trends in atomic radius, electronegativity, electron affinity, melting and boiling points, and solubility, including a discussion of. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID5949337 Boiling Point Properties Of Halogens This is because it has the weakest van der waals. Fluorine has the lowest melting and boiling points in group 7 and is therefore the most volatile. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Predict the melting and boiling points of astatine, and its state at room temperature. Each molecule contains. Boiling Point Properties Of Halogens.
From chempedia.info
Boiling point of halogens Big Chemical Encyclopedia Boiling Point Properties Of Halogens There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. We can explain this trend by looking at. Melting. Boiling Point Properties Of Halogens.
From wwwchem.uwimona.edu.jm
The Chemistry of the Halogens 2 Boiling Point Properties Of Halogens There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the. This is because it has the weakest van der waals. Predict the melting and boiling points of astatine, and its state at room temperature. Sections below cover the trends in atomic radius, electronegativity, electron affinity,. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2319114 Boiling Point Properties Of Halogens There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the. We can explain this trend by looking at. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. The halogens exist as simple. It can be seen that there. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Boiling Point Properties Of Halogens The halogens exist as simple. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Astatine should have a melting point of about 300°c and a boiling point of about 340°c. Each molecule contains two halogen. Predict the melting and boiling points of astatine,. Boiling Point Properties Of Halogens.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2319114 Boiling Point Properties Of Halogens Boiling point increases down the group. Each molecule contains two halogen. This is because it has the weakest van der waals. Melting and boiling points (increase down the group) the melting and boiling points increase down the group because of the van der waals. Fluorine has the lowest melting and boiling points in group 7 and is therefore the most. Boiling Point Properties Of Halogens.