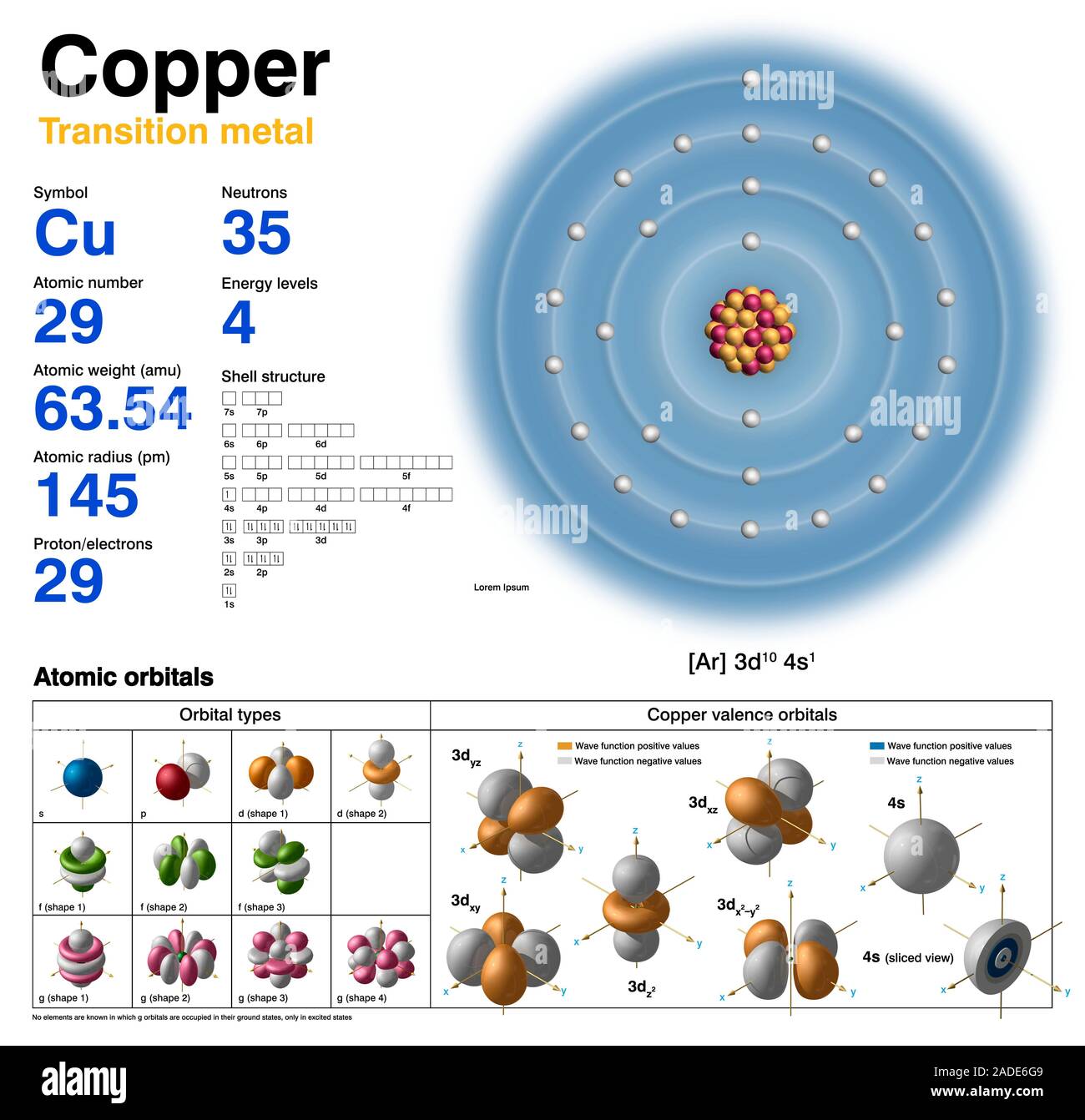
Copper Electron Configuration Aufbau . In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The actual electron configuration may be. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. The aufbau principle works for nearly every element tested. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. There are two exceptions to this principle, chromium, and copper.
from www.alamy.com
The actual electron configuration may be. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. There are two exceptions to this principle, chromium, and copper. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The aufbau principle works for nearly every element tested. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ.
Copper (Cu). Diagram of the nuclear composition, electron configuration
Copper Electron Configuration Aufbau For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The aufbau principle works for nearly every element tested. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The actual electron configuration may be. There are two exceptions to this principle, chromium, and copper.
From www.periodic-table.org
Copper Electron Configuration and Oxidation States Cu Copper Electron Configuration Aufbau In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. The actual electron configuration may be. How to write the electron configuration for. Copper Electron Configuration Aufbau.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Aufbau How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. Similarly, the observed electron configuration of copper is [ar]4s 1 3d. Copper Electron Configuration Aufbau.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electron Configuration Aufbau In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. The actual electron configuration may be. The aufbau principle works for nearly every element tested. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. For example, the. Copper Electron Configuration Aufbau.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Aufbau How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. 1s 2 2s 2 2p 6 3 s 2 3p 6. Copper Electron Configuration Aufbau.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Configuration Aufbau The actual electron configuration may be. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. There are two exceptions to this principle, chromium, and copper. How to write. Copper Electron Configuration Aufbau.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Electron Configuration Aufbau There are two exceptions to this principle, chromium, and copper. The actual electron configuration may be. The aufbau principle works for nearly every element tested. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. How to write the electron configuration for copper (cu, cu+, and cu2+). Copper Electron Configuration Aufbau.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Copper Electron Configuration Aufbau Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The aufbau principle works for nearly every element tested. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The actual electron configuration may be. In any atom with two or more electrons, the. Copper Electron Configuration Aufbau.
From www.youtube.com
Electronic Configuration of Chromium and Copper Structure of Atom Copper Electron Configuration Aufbau In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. The aufbau principle works for nearly every element tested. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The actual electron configuration may be. Similarly, the observed electron configuration of. Copper Electron Configuration Aufbau.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Copper Electron Configuration Aufbau The aufbau principle works for nearly every element tested. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Similarly, the. Copper Electron Configuration Aufbau.
From www.youtube.com
How to write/find/do the electron configuration of Cr(Chromium) and Cu Copper Electron Configuration Aufbau The actual electron configuration may be. The aufbau principle works for nearly every element tested. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. For example, the observed. Copper Electron Configuration Aufbau.
From periodictable.me
Copper Electron Configuration (Cu) with Orbital Diagram Copper Electron Configuration Aufbau The actual electron configuration may be. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. How to write the electron configuration for copper (cu, cu+, and cu2+) in. Copper Electron Configuration Aufbau.
From mungfali.com
Orbital Diagram Of Copper Copper Electron Configuration Aufbau How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The aufbau principle works for nearly every element tested. Similarly, the observed electron configuration of copper. Copper Electron Configuration Aufbau.
From www.dreamstime.com
Electron of the Element Copper Stock Vector Illustration of Copper Electron Configuration Aufbau In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The actual electron configuration may be. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s. Copper Electron Configuration Aufbau.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Copper Electron Configuration Aufbau Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The actual electron configuration may be. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. How to write the electron configuration for copper (cu, cu+, and cu2+) in. Copper Electron Configuration Aufbau.
From childhealthpolicy.vumc.org
💐 Copper atom model. Copper Particles. 20221017 Copper Electron Configuration Aufbau In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. The actual electron configuration may be. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. 1s 2 2s 2. Copper Electron Configuration Aufbau.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electron Configuration Aufbau There are two exceptions to this principle, chromium, and copper. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. The actual electron configuration may be. Similarly, the. Copper Electron Configuration Aufbau.
From stock.adobe.com
Cu Copper Element Information Facts, Properties, Trends, Uses and Copper Electron Configuration Aufbau There are two exceptions to this principle, chromium, and copper. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. The aufbau principle works for nearly every element tested. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. How to. Copper Electron Configuration Aufbau.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Electron Configuration Aufbau The aufbau principle works for nearly every element tested. There are two exceptions to this principle, chromium, and copper. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. The actual electron configuration may be. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10. Copper Electron Configuration Aufbau.
From www.toppr.com
The electronic configuration of copper (29Cu) is. Copper Electron Configuration Aufbau Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. There are two exceptions to this principle, chromium, and copper. The aufbau principle works for nearly every element. Copper Electron Configuration Aufbau.
From organicful44.blogspot.com
copper orbital diagram Organicful Copper Electron Configuration Aufbau Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The actual electron configuration may be. There are two. Copper Electron Configuration Aufbau.
From mavink.com
Electron Orbital Configuration Chart Copper Electron Configuration Aufbau There are two exceptions to this principle, chromium, and copper. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. How to write the electron configuration for copper (cu, cu+, and. Copper Electron Configuration Aufbau.
From www.alamy.com
3d render of atom structure of copper isolated over white background Copper Electron Configuration Aufbau 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. The actual electron. Copper Electron Configuration Aufbau.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Copper (Cu) YouTube Copper Electron Configuration Aufbau The aufbau principle works for nearly every element tested. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. Similarly, the observed electron configuration of copper is [ar]4s. Copper Electron Configuration Aufbau.
From www.youtube.com
ELECTRONIC CONFIGURATION OF COPPER ATOM and STABILITY YouTube Copper Electron Configuration Aufbau Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9.. Copper Electron Configuration Aufbau.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Configuration Aufbau 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The actual electron configuration may be. In any atom with two or more electrons, the repulsion between the electrons. Copper Electron Configuration Aufbau.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Copper Electron Configuration Aufbau Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. For example, the observed ground state electron configuration of. Copper Electron Configuration Aufbau.
From www.youtube.com
Exceptions to Aufbau principle Chromium and Copper configuration BSc Copper Electron Configuration Aufbau How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The. Copper Electron Configuration Aufbau.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Aufbau The aufbau principle works for nearly every element tested. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. The actual electron configuration may be. Similarly, the observed. Copper Electron Configuration Aufbau.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electron Configuration Aufbau The actual electron configuration may be. There are two exceptions to this principle, chromium, and copper. The aufbau principle works for nearly every element tested. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. In any atom with two or more electrons, the repulsion between the. Copper Electron Configuration Aufbau.
From www.earthdate.org
Copper’s Superpower EarthDate Copper Electron Configuration Aufbau For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \. Copper Electron Configuration Aufbau.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Electron Configuration Aufbau For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Similarly, the observed electron. Copper Electron Configuration Aufbau.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Electron Configuration Aufbau How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. The actual electron configuration may be. 1s 2 2s 2 2p. Copper Electron Configuration Aufbau.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Copper Electron Configuration Aufbau How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. Similarly, the observed electron configuration of copper is [ar]4s 1. Copper Electron Configuration Aufbau.
From www.alamy.com
Copper (Cu). Diagram of the valence orbitals of an atom of copper64 Copper Electron Configuration Aufbau The actual electron configuration may be. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \ (l\) differ. Similarly, the observed electron configuration of copper is [ar]4s 1 3d 10. Copper Electron Configuration Aufbau.
From www.sciencephoto.com
Copper, atomic structure Stock Image C013/1552 Science Photo Library Copper Electron Configuration Aufbau For example, the observed ground state electron configuration of chromium is [ar]4s 1 3d 5 rather than the predicted [ar]4s 2 3d 4. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \. Copper Electron Configuration Aufbau.