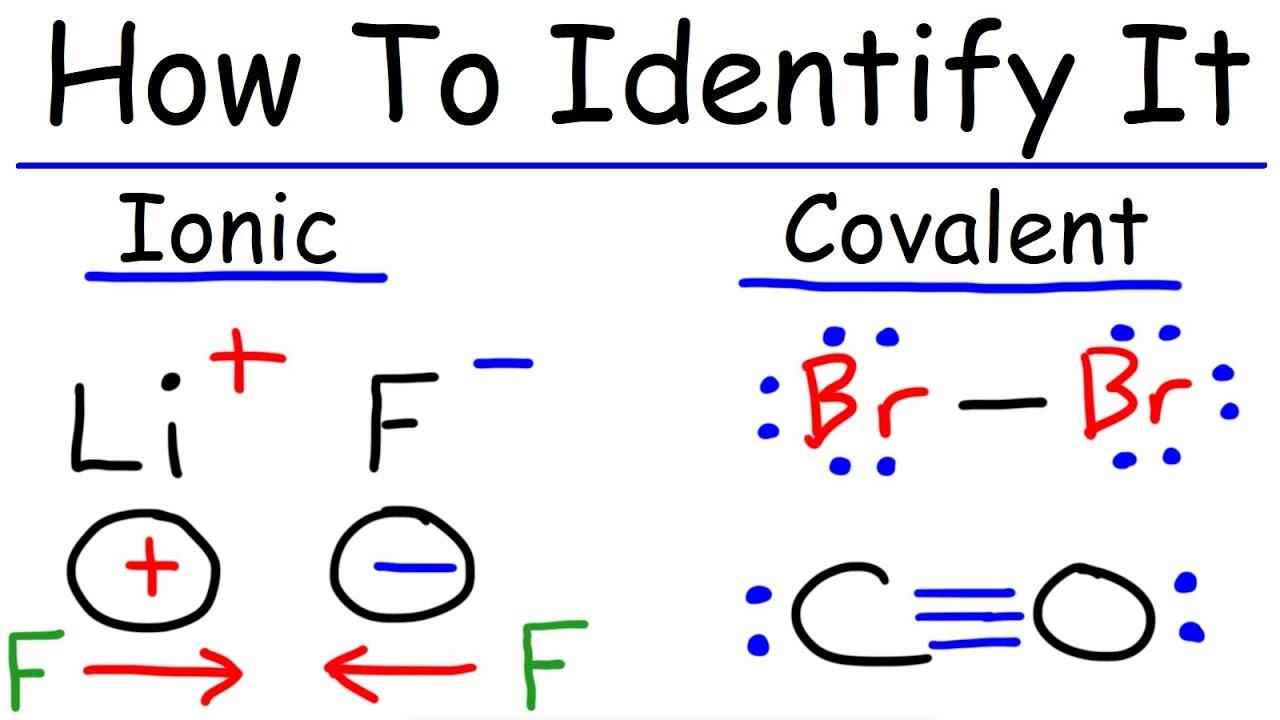
Modeling Ionic And Covalent Bonds 1 Nitrate Anion . a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. chemistry questions and answers. Ionic bonding is also known as. Ionic bonding is the process of not sharing electrons between two atoms. Metal atoms lose electrons to. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. It occurs between a nonmetal and a metal.
from www.youtube.com
ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. Metal atoms lose electrons to. Ionic bonding is the process of not sharing electrons between two atoms. It occurs between a nonmetal and a metal. Ionic bonding is also known as. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. chemistry questions and answers. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun.
Ionic and Covalent Bonding Chemistry YouTube
Modeling Ionic And Covalent Bonds 1 Nitrate Anion chemistry questions and answers. Ionic bonding is also known as. chemistry questions and answers. Ionic bonding is the process of not sharing electrons between two atoms. It occurs between a nonmetal and a metal. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. Metal atoms lose electrons to. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Modeling Ionic And Covalent Bonds 1 Nitrate Anion submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. Metal atoms lose electrons to. It occurs between a nonmetal and a metal. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. chemistry questions and. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary Modeling Ionic And Covalent Bonds 1 Nitrate Anion submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. chemistry questions and answers. Metal atoms lose electrons to. Ionic bonding is also known as. It occurs between a nonmetal and a metal. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.chegg.com
Solved Modeling lonic and Covalent Bonds 2 In each box, Modeling Ionic And Covalent Bonds 1 Nitrate Anion chemistry questions and answers. Metal atoms lose electrons to. submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. It occurs between a nonmetal and a metal. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From thisonevsthatone.com
Ionic vs Covalent Which is which and how to tell them apart Modeling Ionic And Covalent Bonds 1 Nitrate Anion ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Metal atoms lose electrons to. Ionic bonding is the process of not sharing electrons between two atoms. It occurs between a nonmetal. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From socratic.org
What structural units make up ionic solids? Socratic Modeling Ionic And Covalent Bonds 1 Nitrate Anion Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. Ionic bonding is also known as. Ionic bonding is the process of not sharing electrons between two atoms. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Metal atoms lose electrons. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.coursehero.com
[Solved] Modeling Tonic and Covalent Bonds 1 In each box, enter the Modeling Ionic And Covalent Bonds 1 Nitrate Anion It occurs between a nonmetal and a metal. Metal atoms lose electrons to. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. Ionic bonding is the process of not sharing electrons between two atoms. a fundamental difference between compounds containing ionic bonds and those containing covalent. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From schematicerfizyopw.z4.web.core.windows.net
How To Identify A Polar Covalent Bond Modeling Ionic And Covalent Bonds 1 Nitrate Anion Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Ionic bonding is the process of not sharing electrons between two atoms. chemistry questions and answers. It occurs between a nonmetal. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Modeling Ionic And Covalent Bonds 1 Nitrate Anion Ionic bonding is also known as. Metal atoms lose electrons to. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. ionic and covalent bonds are both strong, but unlike covalent. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Modeling Ionic And Covalent Bonds 1 Nitrate Anion It occurs between a nonmetal and a metal. Ionic bonding is the process of not sharing electrons between two atoms. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. ionic and. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From sciencenotes.org
Compounds With Both Ionic and Covalent Bonds Modeling Ionic And Covalent Bonds 1 Nitrate Anion Metal atoms lose electrons to. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. Ionic bonding is the process of not sharing electrons between two atoms. chemistry questions and answers. Ionic bonding is also known as. a fundamental difference between compounds containing ionic bonds and. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From courses.lumenlearning.com
Ionic Radius Introduction to Chemistry Modeling Ionic And Covalent Bonds 1 Nitrate Anion Metal atoms lose electrons to. It occurs between a nonmetal and a metal. Ionic bonding is the process of not sharing electrons between two atoms. chemistry questions and answers. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. a fundamental difference between compounds containing ionic. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Modeling Ionic And Covalent Bonds 1 Nitrate Anion submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From ar.inspiredpencil.com
Ionic Bond Dot Diagram Modeling Ionic And Covalent Bonds 1 Nitrate Anion submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. Ionic bonding is also known as. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. Modeling lonic and covalent bonds 2 in each box, enter the. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Modeling Ionic And Covalent Bonds 1 Nitrate Anion Ionic bonding is also known as. submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. It occurs between a nonmetal and a metal. Ionic bonding is the process of not sharing electrons between two atoms. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.youtube.com
Ionic and Covalent Bonding Chemistry YouTube Modeling Ionic And Covalent Bonds 1 Nitrate Anion Ionic bonding is the process of not sharing electrons between two atoms. chemistry questions and answers. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. It occurs between a nonmetal. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.chegg.com
Solved Modeling lonic and Covalent Bonds 2 In each box, Modeling Ionic And Covalent Bonds 1 Nitrate Anion It occurs between a nonmetal and a metal. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. Ionic bonding is the process of not sharing electrons between two atoms. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Metal atoms. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.animalia-life.club
Covalent Compounds Modeling Ionic And Covalent Bonds 1 Nitrate Anion It occurs between a nonmetal and a metal. submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. chemistry questions and answers. Metal atoms lose electrons. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From dxogyodiq.blob.core.windows.net
Bromine Is Anion Or Cation at Glenn Kirby blog Modeling Ionic And Covalent Bonds 1 Nitrate Anion chemistry questions and answers. Ionic bonding is the process of not sharing electrons between two atoms. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. Ionic bonding is also known as. Metal atoms lose electrons to. a fundamental difference between compounds containing ionic bonds and. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From sciencenotes.org
Ionic vs Covalent Bonds Modeling Ionic And Covalent Bonds 1 Nitrate Anion submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. chemistry questions and answers. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Ionic bonding is the process of not sharing electrons between two atoms. Metal atoms lose electrons to. Ionic. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.teachit.co.uk
Covalent & ionic bonding models KS4 chemistry Teachit Modeling Ionic And Covalent Bonds 1 Nitrate Anion Metal atoms lose electrons to. Ionic bonding is the process of not sharing electrons between two atoms. Ionic bonding is also known as. chemistry questions and answers. It occurs between a nonmetal and a metal. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Modeling lonic and covalent bonds 2 in each. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.youtube.com
Nitrate Ion Lewis Structure How to Draw the Lewis Structure for Modeling Ionic And Covalent Bonds 1 Nitrate Anion a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. It occurs between a nonmetal and a metal. submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Modeling Ionic And Covalent Bonds 1 Nitrate Anion submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. chemistry questions and answers. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. Metal atoms lose electrons to. It occurs between a nonmetal and a. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Modeling Ionic And Covalent Bonds 1 Nitrate Anion Ionic bonding is the process of not sharing electrons between two atoms. Metal atoms lose electrons to. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. Ionic bonding is also known as. chemistry questions and answers. It occurs between a nonmetal and a metal. Modeling lonic. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.youtube.com
Resonance Structures for NO3 (Nitrate Ion) YouTube Modeling Ionic And Covalent Bonds 1 Nitrate Anion Ionic bonding is also known as. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. chemistry questions and answers. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. It occurs between a nonmetal and a metal. Modeling lonic and. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.numerade.com
SOLVED 'Modeling Ionic and Covalent Bonds 1 In each box, enter the Modeling Ionic And Covalent Bonds 1 Nitrate Anion Ionic bonding is the process of not sharing electrons between two atoms. submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. chemistry questions and answers. Modeling lonic and covalent bonds 2. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Modeling Ionic And Covalent Bonds 1 Nitrate Anion Ionic bonding is the process of not sharing electrons between two atoms. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. chemistry questions and answers. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From pediaa.com
Difference Between Covalent and Ionic Bonds Modeling Ionic And Covalent Bonds 1 Nitrate Anion Metal atoms lose electrons to. It occurs between a nonmetal and a metal. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. submit your choice when you are confident you. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts Modeling Ionic And Covalent Bonds 1 Nitrate Anion Ionic bonding is also known as. It occurs between a nonmetal and a metal. submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Metal atoms lose electrons to. Ionic bonding is the. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Modeling Ionic And Covalent Bonds 1 Nitrate Anion a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Ionic bonding is also known as. It occurs between a nonmetal and a metal. chemistry questions and answers. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. submit your. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From en.wikipedia.org
Ionic bonding Wikipedia Modeling Ionic And Covalent Bonds 1 Nitrate Anion submit your choice when you are confident you have the correct answer.nitrate anion modeling ionic and covalent bonds in. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. chemistry questions and answers. Modeling lonic and covalent bonds 2 in each box, enter the appropriate. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.numerade.com
SOLVED Modeling Ionic and Covalent Bonds 2 In each box, enter the Modeling Ionic And Covalent Bonds 1 Nitrate Anion ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. It occurs between a nonmetal and a metal. Ionic bonding is the process of not sharing electrons between two atoms. Ionic bonding. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From coredifferences.com
15 Major Difference between Covalent and Ionic Bonds with Table Core Modeling Ionic And Covalent Bonds 1 Nitrate Anion It occurs between a nonmetal and a metal. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. Ionic bonding is the process of not sharing electrons between two atoms. chemistry questions and answers. Ionic bonding is also known as. Modeling lonic and covalent bonds 2 in each box, enter the appropriate number. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.teachoo.com
[Class 10] Differentiate between Ionic bond & Covalent bond Teachoo Modeling Ionic And Covalent Bonds 1 Nitrate Anion a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. chemistry questions and answers. ionic and covalent bonds are both strong, but unlike covalent bonds, ionic bonds are not directed at only one other atom. Ionic bonding is the process of not sharing electrons between two atoms. Modeling lonic and covalent bonds. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From www.coursehero.com
[Solved] New Modeling Ionic and Covalent Bonds 1 In each box, enter the Modeling Ionic And Covalent Bonds 1 Nitrate Anion Ionic bonding is the process of not sharing electrons between two atoms. chemistry questions and answers. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. It occurs between a nonmetal and a metal. Metal atoms lose electrons to. submit your choice when you are confident you have the correct answer.nitrate anion. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.
From schematicempresssgdv.z22.web.core.windows.net
Energy Diagram Covalent Bond Modeling Ionic And Covalent Bonds 1 Nitrate Anion Modeling lonic and covalent bonds 2 in each box, enter the appropriate number of valence electrons for each atom and the nun. It occurs between a nonmetal and a metal. a fundamental difference between compounds containing ionic bonds and those containing covalent bonds is the. chemistry questions and answers. Ionic bonding is also known as. Metal atoms lose. Modeling Ionic And Covalent Bonds 1 Nitrate Anion.