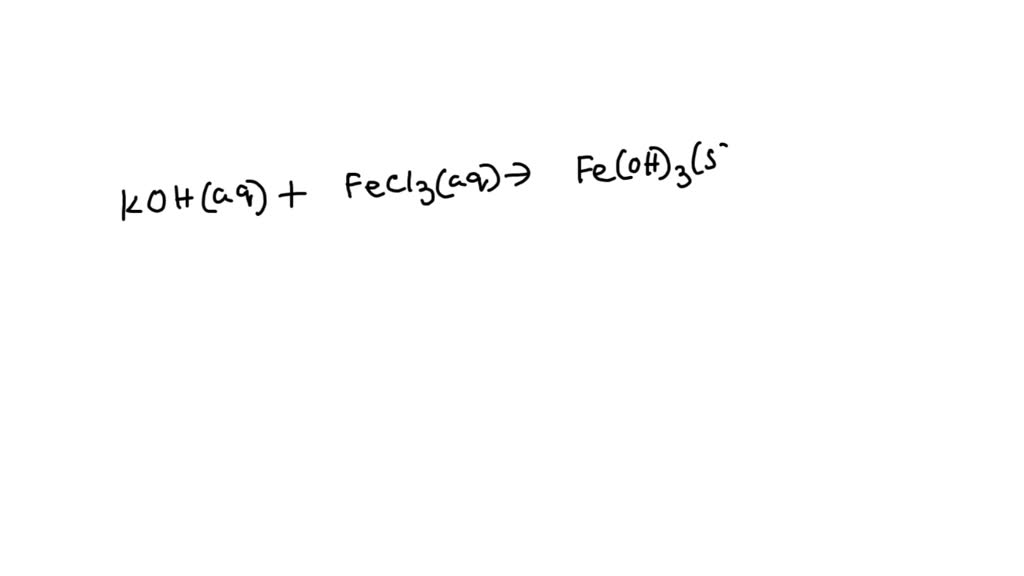Iron (Iii) Chloride + Potassium Phosphate . Write and balance the molecular, ionic, and net ionic equations. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. Enter an equation of an. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. iron(ii) chloride and potassium phosphate react. use the calculator below to balance chemical equations and determine the type of reaction (instructions).
from www.numerade.com
when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. use the calculator below to balance chemical equations and determine the type of reaction (instructions). enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Enter an equation of an. iron(ii) chloride and potassium phosphate react. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. Write and balance the molecular, ionic, and net ionic equations. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the.
SOLVED Write the balanced chemical equation for the reaction of
Iron (Iii) Chloride + Potassium Phosphate Enter an equation of an. iron(ii) chloride and potassium phosphate react. Write and balance the molecular, ionic, and net ionic equations. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. use the calculator below to balance chemical equations and determine the type of reaction (instructions). enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. Enter an equation of an. when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution.
From giolegnki.blob.core.windows.net
Chlorine Phosphate Chemical Formula at Michael Valenti blog Iron (Iii) Chloride + Potassium Phosphate when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. use the calculator below to balance chemical equations and determine the type of reaction (instructions). Write and balance the molecular, ionic, and net ionic equations. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. use the calculator below to balance chemical equations and determine the type of reaction (instructions). when aqueous solutions of potassium phosphate and iron(iii) chloride. Iron (Iii) Chloride + Potassium Phosphate.
From www.chegg.com
Solved Iron(III) chloride + Potassium phosphate Yellowbrown Iron (Iii) Chloride + Potassium Phosphate when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. learn about and revise ionic compounds with. Iron (Iii) Chloride + Potassium Phosphate.
From www.chegg.com
Solved (4pts) For the reaction of iron(III) chloride and Iron (Iii) Chloride + Potassium Phosphate use the calculator below to balance chemical equations and determine the type of reaction (instructions). potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms. Iron (Iii) Chloride + Potassium Phosphate.
From www.youtube.com
How to Write the Formula for Potassium phosphate YouTube Iron (Iii) Chloride + Potassium Phosphate Write and balance the molecular, ionic, and net ionic equations. when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. Enter an equation of an. enter an ionic equation (solubility states are optional) to. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate Enter an equation of an. Write and balance the molecular, ionic, and net ionic equations. iron(ii) chloride and potassium phosphate react. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. learn about and. Iron (Iii) Chloride + Potassium Phosphate.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Iron (Iii) Chloride + Potassium Phosphate potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. iron(ii) chloride and potassium phosphate react. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. Write and balance the molecular, ionic, and net ionic equations. Enter an equation of an. when aqueous solutions. Iron (Iii) Chloride + Potassium Phosphate.
From exombjkja.blob.core.windows.net
Iron 3 Chloride Ligands at Linda Chase blog Iron (Iii) Chloride + Potassium Phosphate enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. Write and balance the molecular, ionic, and net ionic equations. iron(ii) chloride and potassium phosphate react. use the calculator below to balance chemical. Iron (Iii) Chloride + Potassium Phosphate.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Iron (Iii) Chloride + Potassium Phosphate enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. use the calculator below to balance chemical equations and determine the type of reaction (instructions). 1 fecl 3 + 1 k 3 po 4. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. use the calculator below to balance chemical equations and determine the type of reaction (instructions). when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. iron(ii) chloride and potassium phosphate react. learn. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. Enter an equation of an. Write and balance the molecular, ionic, and net ionic equations. learn about and revise ionic compounds with this bbc. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. 1 fecl 3 + 1 k 3. Iron (Iii) Chloride + Potassium Phosphate.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Iron (Iii) Chloride + Potassium Phosphate when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. Write and balance the molecular, ionic, and net ionic equations. iron(ii) chloride and potassium phosphate react. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. 1 fecl 3 + 1 k 3. Iron (Iii) Chloride + Potassium Phosphate.
From www.youtube.com
2Fe(NO3)3 + 3K2HPO₄ → Fe2(HPO4)3↓ + 6KNO3 Potassium phosphate +Iron Iron (Iii) Chloride + Potassium Phosphate Enter an equation of an. Write and balance the molecular, ionic, and net ionic equations. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. when aqueous solutions of potassium phosphate and iron(iii) chloride are. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. use the calculator below to balance chemical equations and determine the type of reaction (instructions). iron(ii) chloride and potassium phosphate react. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element,. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. Write and balance the molecular, ionic, and net ionic. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate iron(ii) chloride and potassium phosphate react. when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. . Iron (Iii) Chloride + Potassium Phosphate.
From exombjkja.blob.core.windows.net
Iron 3 Chloride Ligands at Linda Chase blog Iron (Iii) Chloride + Potassium Phosphate 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. Enter an equation of an. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. enter an ionic. Iron (Iii) Chloride + Potassium Phosphate.
From www.shutterstock.com
6,181 Calcium Phosphorus Food Images, Stock Photos & Vectors Shutterstock Iron (Iii) Chloride + Potassium Phosphate enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. use the calculator below to balance chemical equations. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate Enter an equation of an. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. iron(ii) chloride and potassium phosphate react. Write and balance the molecular, ionic, and net ionic equations. use the calculator below to balance chemical equations and determine the type of reaction (instructions). potassium phosphate, k3po4, reacts. Iron (Iii) Chloride + Potassium Phosphate.
From www.chegg.com
Solved Iron(III) chloride + Potassium phosphate Yellowbrown Iron (Iii) Chloride + Potassium Phosphate Enter an equation of an. use the calculator below to balance chemical equations and determine the type of reaction (instructions). potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. iron(ii) chloride and. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. when aqueous solutions of potassium phosphate and iron(iii). Iron (Iii) Chloride + Potassium Phosphate.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation ID6658952 Iron (Iii) Chloride + Potassium Phosphate Enter an equation of an. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. use the calculator below to balance chemical equations and determine the type of reaction (instructions). 1 fecl 3 +. Iron (Iii) Chloride + Potassium Phosphate.
From www.youtube.com
How to Balance CaCl2 + K3PO4 = Ca3(PO4)2 + KCl (Calcium chloride Iron (Iii) Chloride + Potassium Phosphate Enter an equation of an. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. use the calculator below to balance chemical equations and determine the type of reaction (instructions). enter an ionic equation. Iron (Iii) Chloride + Potassium Phosphate.
From www.coursehero.com
[Solved] Aqueous potassium phosphate reacts with aqueous aluminum Iron (Iii) Chloride + Potassium Phosphate enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. potassium phosphate, k3po4, reacts with iron (iii) chloride,. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate use the calculator below to balance chemical equations and determine the type of reaction (instructions). Write and balance the molecular, ionic, and net ionic equations. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the.. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. Enter an equation of an. potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. use the. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. Enter an equation of an. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. iron(ii) chloride and. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate Enter an equation of an. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. iron(ii) chloride and potassium phosphate react. potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. use the calculator below to balance chemical equations and determine the type of. Iron (Iii) Chloride + Potassium Phosphate.
From www.slideserve.com
PPT Writing Ionic Formulas PowerPoint Presentation, free download Iron (Iii) Chloride + Potassium Phosphate when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. Write and balance the molecular, ionic, and net ionic equations. iron(ii) chloride and potassium phosphate react. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of. Iron (Iii) Chloride + Potassium Phosphate.
From www.chegg.com
Solved Gold (i) chloride and potassium phosphate Reaction Iron (Iii) Chloride + Potassium Phosphate 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. Enter an equation of an. potassium phosphate, k3po4, reacts with iron (iii) chloride, fecl3, in aqueous solution to form a precipitate. learn about and. Iron (Iii) Chloride + Potassium Phosphate.
From www.youtube.com
Iron III Chloride Reaction With Potassium Thiocyanate (FeCl3 + KSCN Iron (Iii) Chloride + Potassium Phosphate enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. 1 fecl 3 + 1 k 3 po 4 = 1 kcl + 1 fepo 4 for each element, we check if the number of atoms is balanced on both sides of the. learn about and revise ionic compounds with this. Iron (Iii) Chloride + Potassium Phosphate.
From chemistry-chemists.com
Iron (II), (III) salts, ferrocyanide and ferricyanide. Соли железа (II Iron (Iii) Chloride + Potassium Phosphate when aqueous solutions of potassium phosphate and iron(iii) chloride are combined, solid iron(iii) phosphate and a solution. learn about and revise ionic compounds with this bbc bitesize gcse combined science (edexcel) study guide. iron(ii) chloride and potassium phosphate react. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Write. Iron (Iii) Chloride + Potassium Phosphate.