Zinc Copper Battery Anode Cathode Reactions . The copper cations on the. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. the anode is where oxidation occurs, and the cathode is the site of reduction. at the anode, the zinc atoms lose electrons, which leave the battery through the wires. The $\ce{zn}$ screw acted as the cathode, and the. In an actual cell, the identity of the electrodes depends on the direction. i prepared a battery with $\ce{zn}$ screws and a $\ce{cu}$ wire, with water as an electrolyte. in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. The zinc ions that form enter the solution. the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+.
from stock.adobe.com
the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. at the anode, the zinc atoms lose electrons, which leave the battery through the wires. The zinc ions that form enter the solution. The $\ce{zn}$ screw acted as the cathode, and the. In an actual cell, the identity of the electrodes depends on the direction. i prepared a battery with $\ce{zn}$ screws and a $\ce{cu}$ wire, with water as an electrolyte. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. The copper cations on the. the anode is where oxidation occurs, and the cathode is the site of reduction.
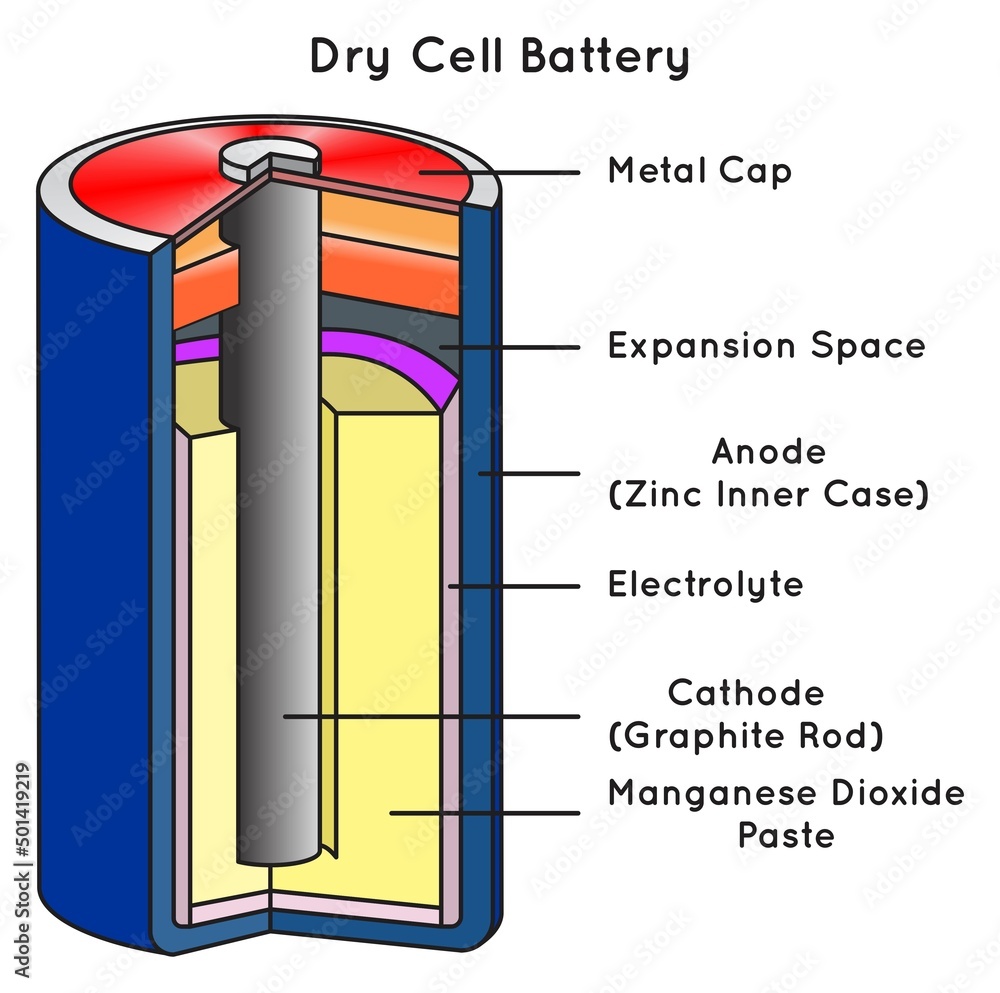
Stockvector Dry cell battery infographic diagram parts structure anode
Zinc Copper Battery Anode Cathode Reactions The zinc ions that form enter the solution. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. i prepared a battery with $\ce{zn}$ screws and a $\ce{cu}$ wire, with water as an electrolyte. In an actual cell, the identity of the electrodes depends on the direction. at the anode, the zinc atoms lose electrons, which leave the battery through the wires. The $\ce{zn}$ screw acted as the cathode, and the. the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. The copper cations on the. in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. The zinc ions that form enter the solution. the anode is where oxidation occurs, and the cathode is the site of reduction.
From stock.adobe.com
Stockvector Dry cell battery infographic diagram parts structure anode Zinc Copper Battery Anode Cathode Reactions the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. The $\ce{zn}$ screw acted as the cathode, and the. i prepared a battery with $\ce{zn}$ screws and a $\ce{cu}$ wire, with water as an electrolyte. the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s). Zinc Copper Battery Anode Cathode Reactions.
From exypbyonv.blob.core.windows.net
What Are Inert Electrodes In Electrolysis at Gene Dickson blog Zinc Copper Battery Anode Cathode Reactions in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. at the anode, the zinc atoms lose electrons, which leave the battery through the wires. The copper cations on the. The $\ce{zn}$ screw acted as the cathode, and the. i prepared a battery with $\ce{zn}$. Zinc Copper Battery Anode Cathode Reactions.
From www.thoughtco.com
How to Define Anode and Cathode Zinc Copper Battery Anode Cathode Reactions The copper cations on the. The zinc ions that form enter the solution. The $\ce{zn}$ screw acted as the cathode, and the. in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. In an actual cell, the identity of the electrodes depends on the direction. the. Zinc Copper Battery Anode Cathode Reactions.
From gioescnmg.blob.core.windows.net
Which Electrochemical Assay Measures Current At Fixed Potential at Zinc Copper Battery Anode Cathode Reactions the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. The zinc ions that form enter the solution. the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. at the anode, the zinc atoms lose electrons, which leave. Zinc Copper Battery Anode Cathode Reactions.
From www.degruyter.com
A review for modified Li composite anode Principle, preparation and Zinc Copper Battery Anode Cathode Reactions in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. The zinc ions that form enter the solution. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. the anode is where oxidation occurs, and the cathode. Zinc Copper Battery Anode Cathode Reactions.
From 2012books.lardbucket.org
Electrolysis Zinc Copper Battery Anode Cathode Reactions in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. The $\ce{zn}$ screw acted as the cathode, and the. at the anode, the zinc atoms lose electrons, which leave the battery through the wires. the two compartments of an electrochemical cell where the half reactions. Zinc Copper Battery Anode Cathode Reactions.
From saylordotorg.github.io
Describing Electrochemical Cells Zinc Copper Battery Anode Cathode Reactions The copper cations on the. at the anode, the zinc atoms lose electrons, which leave the battery through the wires. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. The $\ce{zn}$ screw acted as the cathode, and the. the total electrochemical reaction on the cathode and anode could. Zinc Copper Battery Anode Cathode Reactions.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Zinc Copper Battery Anode Cathode Reactions at the anode, the zinc atoms lose electrons, which leave the battery through the wires. the anode is where oxidation occurs, and the cathode is the site of reduction. in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. The copper cations on the. In. Zinc Copper Battery Anode Cathode Reactions.
From fyodaxmyb.blob.core.windows.net
Anode Cathode Electrolyte at Felisha Jackson blog Zinc Copper Battery Anode Cathode Reactions the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. The copper cations on the. the two compartments of an electrochemical cell where the. Zinc Copper Battery Anode Cathode Reactions.
From www.slideserve.com
PPT OxidationReduction Reactions PowerPoint Presentation, free Zinc Copper Battery Anode Cathode Reactions in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. The $\ce{zn}$ screw acted as the cathode, and the. the anode is where oxidation occurs, and the. Zinc Copper Battery Anode Cathode Reactions.
From www.mdpi.com
Batteries Free FullText AnodeFree Rechargeable SodiumMetal Batteries Zinc Copper Battery Anode Cathode Reactions The zinc ions that form enter the solution. the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. The $\ce{zn}$ screw acted as the cathode, and the. In an actual cell, the identity of the electrodes depends on the direction. the anode is where oxidation occurs, and. Zinc Copper Battery Anode Cathode Reactions.
From www.pinterest.com
enter image description here Chemistry Lesson Plans, Chemistry Zinc Copper Battery Anode Cathode Reactions i prepared a battery with $\ce{zn}$ screws and a $\ce{cu}$ wire, with water as an electrolyte. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. . Zinc Copper Battery Anode Cathode Reactions.
From circuitagolopetzin.z4.web.core.windows.net
What Forms At The Cathode During Electrolysis Zinc Copper Battery Anode Cathode Reactions at the anode, the zinc atoms lose electrons, which leave the battery through the wires. The $\ce{zn}$ screw acted as the cathode, and the. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. the anode is where oxidation occurs, and the cathode is the site of reduction. . Zinc Copper Battery Anode Cathode Reactions.
From giodwrxvk.blob.core.windows.net
Electrodes In Electrolysis at Lindsay Macy blog Zinc Copper Battery Anode Cathode Reactions the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. In an actual cell, the identity of the electrodes depends on the direction. The copper cations on the. the anode is where oxidation occurs, and the cathode is the site of reduction. in the process of. Zinc Copper Battery Anode Cathode Reactions.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Zinc Copper Battery Anode Cathode Reactions In an actual cell, the identity of the electrodes depends on the direction. the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. i prepared a battery with $\ce{zn}$ screws and a $\ce{cu}$ wire, with water as an electrolyte. The $\ce{zn}$ screw acted as the cathode, and. Zinc Copper Battery Anode Cathode Reactions.
From stock.adobe.com
Stockvector Anode and cathode scientific physics education diagram Zinc Copper Battery Anode Cathode Reactions the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. The $\ce{zn}$ screw acted as the cathode, and the. at the anode, the zinc atoms lose electrons, which leave the battery through the wires. the two compartments of an electrochemical cell where the half reactions occur. Zinc Copper Battery Anode Cathode Reactions.
From psu.pb.unizin.org
17.7 Electrolysis Chemistry 112 Chapters 1217 of OpenStax General Zinc Copper Battery Anode Cathode Reactions the anode is where oxidation occurs, and the cathode is the site of reduction. in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. The copper cations on the. In an actual cell, the identity of the electrodes depends on the direction. The $\ce{zn}$ screw acted. Zinc Copper Battery Anode Cathode Reactions.
From chemistry-europe.onlinelibrary.wiley.com
The Anode Materials for Lithium‐Ion and Sodium‐Ion Batteries Based on Zinc Copper Battery Anode Cathode Reactions the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. The copper cations on the. at the anode, the zinc atoms lose electrons, which leave the battery through the wires. i prepared a battery with $\ce{zn}$ screws and a $\ce{cu}$ wire, with water as an electrolyte.. Zinc Copper Battery Anode Cathode Reactions.
From guidewiringstrunting.z14.web.core.windows.net
Cathode Charge In Electrolysis Zinc Copper Battery Anode Cathode Reactions The zinc ions that form enter the solution. the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. the anode is where oxidation occurs, and the cathode is. Zinc Copper Battery Anode Cathode Reactions.
From schematicciricostajj.z4.web.core.windows.net
Cathode Charge In Electrolysis Zinc Copper Battery Anode Cathode Reactions In an actual cell, the identity of the electrodes depends on the direction. the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. at the anode, the zinc atoms lose electrons, which leave the battery through the wires. in the process of the reaction, electrons can. Zinc Copper Battery Anode Cathode Reactions.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry for Majors Zinc Copper Battery Anode Cathode Reactions the total electrochemical reaction on the cathode and anode could be interpreted as [zn(s) + cu 2+ (aq) ⇄ zn 2+. In an actual cell, the identity of the electrodes depends on the direction. i prepared a battery with $\ce{zn}$ screws and a $\ce{cu}$ wire, with water as an electrolyte. The $\ce{zn}$ screw acted as the cathode, and. Zinc Copper Battery Anode Cathode Reactions.
From fixlibrarymarkbladgr.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram Zinc Copper Battery Anode Cathode Reactions In an actual cell, the identity of the electrodes depends on the direction. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. the anode is where oxidation occurs, and the cathode is the site of reduction. The copper cations on the. the total electrochemical reaction on the cathode. Zinc Copper Battery Anode Cathode Reactions.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Zinc Copper Battery Anode Cathode Reactions in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. The copper cations on the. the anode is where oxidation occurs, and the cathode is the site of reduction. the two compartments of an electrochemical cell where the half reactions occur are called the anode. Zinc Copper Battery Anode Cathode Reactions.
From www.zswu.dicp.ac.cn
Recent progress of dendritefree stable zinc anodes for advanced zinc Zinc Copper Battery Anode Cathode Reactions i prepared a battery with $\ce{zn}$ screws and a $\ce{cu}$ wire, with water as an electrolyte. in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. The zinc ions that form enter the solution. the total electrochemical reaction on the cathode and anode could be. Zinc Copper Battery Anode Cathode Reactions.
From fyowclttk.blob.core.windows.net
Zinc Solid Charge at Diane Garcia blog Zinc Copper Battery Anode Cathode Reactions the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. in the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as. the anode is where oxidation occurs, and the cathode is the site of reduction. i prepared. Zinc Copper Battery Anode Cathode Reactions.
From guidemanualcodas.z1.web.core.windows.net
Diagram Of Voltaic Cell Zinc Copper Battery Anode Cathode Reactions the anode is where oxidation occurs, and the cathode is the site of reduction. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. i prepared a battery with $\ce{zn}$ screws and a $\ce{cu}$ wire, with water as an electrolyte. the total electrochemical reaction on the cathode and. Zinc Copper Battery Anode Cathode Reactions.