Expansion Definition Of Gas . Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion of gases is much greater than solids or liquids,. The heat capacity of a body is the quantity of heat. Chapter 8 heat capacity, and the expansion of gases. This empirical relation, formulated by the physicist robert boyle in 1662, states. This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!).
from www.youtube.com
All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion of gases is much greater than solids or liquids,. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. Chapter 8 heat capacity, and the expansion of gases. This empirical relation, formulated by the physicist robert boyle in 1662, states. The heat capacity of a body is the quantity of heat.
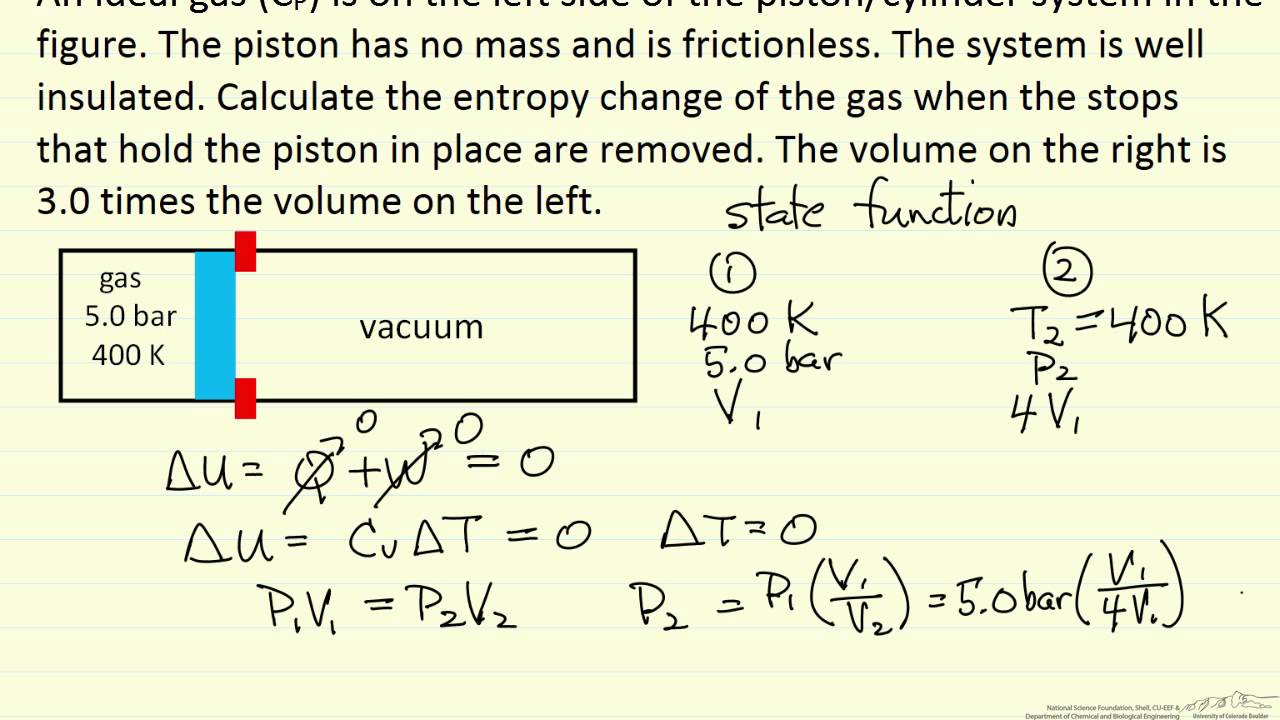
Entropy Change for Ideal Gas Expansion YouTube
Expansion Definition Of Gas The heat capacity of a body is the quantity of heat. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. The heat capacity of a body is the quantity of heat. This empirical relation, formulated by the physicist robert boyle in 1662, states. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion of gases is much greater than solids or liquids,. Chapter 8 heat capacity, and the expansion of gases. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!).
From www.slideserve.com
PPT Heat and the First Law of Thermodynamics PowerPoint Presentation Expansion Definition Of Gas This empirical relation, formulated by the physicist robert boyle in 1662, states. Chapter 8 heat capacity, and the expansion of gases. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). The heat capacity of a body is the quantity of heat. Gas expansion refers to the increase in the volume of a. Expansion Definition Of Gas.
From saylordotorg.github.io
Chemical Thermodynamics Expansion Definition Of Gas Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the physicist robert boyle in 1662, states. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. All three states of matter (solid, liquid and. Expansion Definition Of Gas.
From www.youtube.com
Adiabatic compression and expansion of a gas HD YouTube Expansion Definition Of Gas Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Chapter 8 heat capacity, and the expansion of gases. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. This empirical relation, formulated by the physicist robert boyle in 1662,. Expansion Definition Of Gas.
From studylib.net
Expansion of an Ideal Gas into a Vacuum Expansion Definition Of Gas This empirical relation, formulated by the physicist robert boyle in 1662, states. Chapter 8 heat capacity, and the expansion of gases. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. All three states of matter (solid, liquid and gas) expand when heated, but thermal. Expansion Definition Of Gas.
From readchemistry.com
Adiabatic Expansion of an Ideal Gas Read Chemistry Expansion Definition Of Gas Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. The heat capacity of a body is the quantity of heat. This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. This empirical relation, formulated by the physicist robert boyle in 1662, states. All three states. Expansion Definition Of Gas.
From www.grc.nasa.gov
Isentropic Flow Equations Expansion Definition Of Gas The heat capacity of a body is the quantity of heat. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. This empirical relation, formulated by the physicist robert boyle in 1662, states. Boyle’s law, a relation concerning the compression and expansion of a gas. Expansion Definition Of Gas.
From www.slideserve.com
PPT Applications of the First Law Chapter 3 PowerPoint Presentation Expansion Definition Of Gas Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Chapter 8 heat capacity, and the expansion of gases. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. The heat capacity of a body is the quantity. Expansion Definition Of Gas.
From www.nagwa.com
Question Video Calculating the Temperature of a Gas after an Expansion Expansion Definition Of Gas This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. The heat capacity of a body is the quantity. Expansion Definition Of Gas.
From www.youtube.com
Thermal Expansion & Contraction of Liquids and Gases Oxford Secondary Expansion Definition Of Gas This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. Chapter 8 heat capacity, and the expansion of gases. This empirical relation, formulated by the physicist robert boyle in 1662, states. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in. Expansion Definition Of Gas.
From www.britannica.com
Gas Definition, State of Matter, Properties, Structure, & Facts Expansion Definition Of Gas This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. This empirical relation, formulated by the physicist robert boyle in 1662, states. Boyle’s law, a relation. Expansion Definition Of Gas.
From www.youtube.com
Expansion of Gases Charles Law Heat Part 7 YouTube Expansion Definition Of Gas This empirical relation, formulated by the physicist robert boyle in 1662, states. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). The heat capacity of a body is the quantity of heat. Chapter 8 heat capacity, and. Expansion Definition Of Gas.
From www.youtube.com
During an expansion process the pressure of a gas changes from 15 to Expansion Definition Of Gas This empirical relation, formulated by the physicist robert boyle in 1662, states. Chapter 8 heat capacity, and the expansion of gases. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly. Expansion Definition Of Gas.
From www.tec-science.com
Free adiabatic expansion of an ideal gas in a vacuum tecscience Expansion Definition Of Gas Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. Chapter 8 heat capacity, and the expansion of gases. Gas expansion refers to the increase in the volume of a gas when it is. Expansion Definition Of Gas.
From shaunmwilliams.com
Chapter 16 Presentation Expansion Definition Of Gas The heat capacity of a body is the quantity of heat. Chapter 8 heat capacity, and the expansion of gases. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. This empirical relation, formulated by the physicist robert boyle in 1662, states. Gases are very. Expansion Definition Of Gas.
From www.aplustopper.com
Expansion and Contraction in Solids, Liquids and Gases A Plus Topper Expansion Definition Of Gas All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion of gases is much greater than solids or liquids,. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). This empirical relation, formulated by the physicist robert boyle in 1662, states. Chapter 8 heat capacity, and the expansion. Expansion Definition Of Gas.
From studylib.net
Temperature, Thermal Expansion and the Ideal Gas Law Expansion Definition Of Gas Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). The heat capacity of a body is the quantity of heat. This empirical relation, formulated by the physicist robert boyle in 1662, states. This shows the expansion of. Expansion Definition Of Gas.
From www.slideserve.com
PPT The Adiabatic Expansion of an Ideal Gas PowerPoint Presentation Expansion Definition Of Gas Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. The heat capacity of a body is the quantity of heat. All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion of gases is much greater than solids or liquids,.. Expansion Definition Of Gas.
From www.youtube.com
Expansion of Gases, General Science Lecture Sabaq.pk YouTube Expansion Definition Of Gas All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion of gases is much greater than solids or liquids,. The heat capacity of a body is the quantity of heat. This empirical relation, formulated by the physicist robert boyle in 1662, states. Gas expansion refers to the increase in the volume of a gas when. Expansion Definition Of Gas.
From www.slideshare.net
Gas expansion Expansion Definition Of Gas The heat capacity of a body is the quantity of heat. All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion of gases is much greater than solids or liquids,. This empirical relation, formulated by the physicist robert boyle in 1662, states. Boyle’s law, a relation concerning the compression and expansion of a gas at. Expansion Definition Of Gas.
From www.youtube.com
Entropy Change for Ideal Gas Expansion YouTube Expansion Definition Of Gas This empirical relation, formulated by the physicist robert boyle in 1662, states. The heat capacity of a body is the quantity of heat. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. Boyle’s law, a relation concerning the compression and expansion of a gas. Expansion Definition Of Gas.
From www.youtube.com
Real Gas Expansion YouTube Expansion Definition Of Gas This empirical relation, formulated by the physicist robert boyle in 1662, states. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Chapter 8 heat capacity, and the. Expansion Definition Of Gas.
From eschool.iaspaper.net
What is a Gas Introduction & Modify Eschool Expansion Definition Of Gas Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. The heat capacity of a body is the quantity of heat. Chapter 8 heat capacity, and the expansion of gases.. Expansion Definition Of Gas.
From www.slideserve.com
PPT Chapter 19 The Theory of Gases PowerPoint Presentation Expansion Definition Of Gas Chapter 8 heat capacity, and the expansion of gases. This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion. Expansion Definition Of Gas.
From www.youtube.com
How to find the expansion and compression coefficients for an ideal gas Expansion Definition Of Gas Chapter 8 heat capacity, and the expansion of gases. This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. The heat capacity of a body is the quantity of heat. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the. Expansion Definition Of Gas.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID6150835 Expansion Definition Of Gas Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the physicist robert boyle in 1662, states. This shows the expansion of gas at constant temperature against weight of an object's mass (m). Expansion Definition Of Gas.
From www.aplustopper.com
Expansion and Contraction in Solids, Liquids and Gases A Plus Topper Expansion Definition Of Gas The heat capacity of a body is the quantity of heat. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the physicist robert boyle in 1662, states. This shows the expansion of. Expansion Definition Of Gas.
From www.slideserve.com
PPT Chapter 1 Temperature and Heat PowerPoint Presentation, free Expansion Definition Of Gas All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion of gases is much greater than solids or liquids,. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. This empirical relation, formulated by the physicist robert boyle in 1662,. Expansion Definition Of Gas.
From www.youtube.com
For an ideal gas `PT^(11)` = constant then volume expansion coefficient Expansion Definition Of Gas Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). This shows the. Expansion Definition Of Gas.
From www.youtube.com
Expansion of Gases Class 10 I Learn with BYJU'S YouTube Expansion Definition Of Gas Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion of gases is much greater than solids or liquids,. This empirical. Expansion Definition Of Gas.
From www.slideserve.com
PPT The Second Law of Thermodynamics PowerPoint Presentation, free Expansion Definition Of Gas The heat capacity of a body is the quantity of heat. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. This empirical relation, formulated by the physicist. Expansion Definition Of Gas.
From curiophysics.com
Thermal Expansion Of Gases » Curio Physics Expansion Definition Of Gas This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. Chapter 8 heat capacity, and the expansion of gases. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature.. Expansion Definition Of Gas.
From chem.libretexts.org
19.2 The Second Law of Thermodynamics Chemistry LibreTexts Expansion Definition Of Gas The heat capacity of a body is the quantity of heat. This shows the expansion of gas at constant temperature against weight of an object's mass (m) on the piston. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Boyle’s law, a relation concerning the compression and expansion of a gas at. Expansion Definition Of Gas.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica Expansion Definition Of Gas Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in. This empirical relation, formulated by the physicist robert boyle in 1662, states. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). The heat capacity of a body. Expansion Definition Of Gas.
From studylib.net
The Adiabatic Expansion of Gases Entropy and the Second Law of Expansion Definition Of Gas Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). The heat capacity of a body is the quantity of heat. Gas expansion refers to the increase in the volume of a gas when it is subjected to. Expansion Definition Of Gas.
From www.youtube.com
Find coefficient of volume expansion Theory of Gases Visual Expansion Definition Of Gas The heat capacity of a body is the quantity of heat. All three states of matter (solid, liquid and gas) expand when heated, but thermal expansion of gases is much greater than solids or liquids,. Gas expansion refers to the increase in the volume of a gas when it is subjected to a decrease in pressure or an increase in.. Expansion Definition Of Gas.