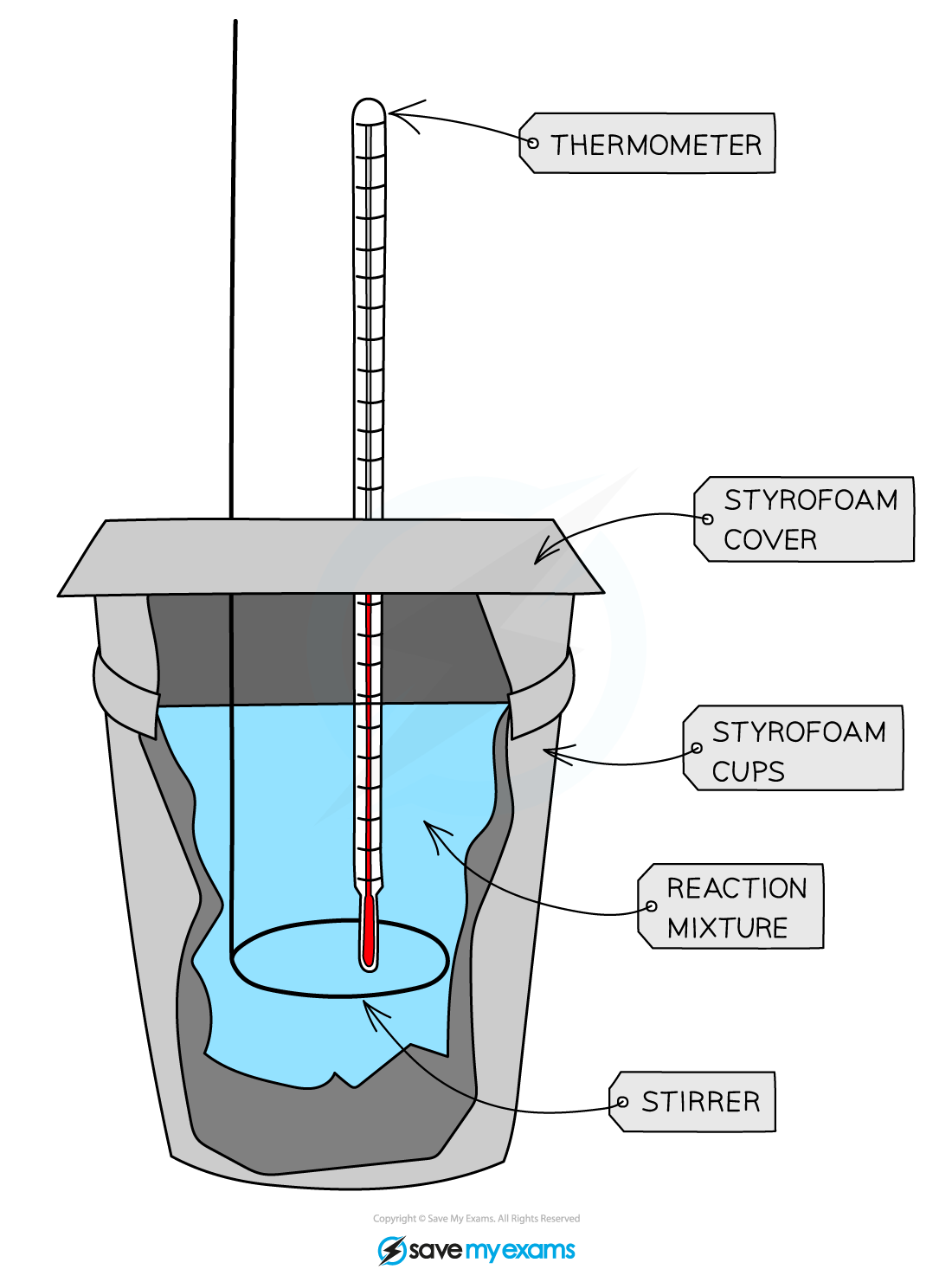
Calorimetry Igcse Chemistry . To do so, the heat is exchanged with a calibrated object. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Calorimetry allows for the measurement of the amount of. We can experimentally determine the relative amounts of energy released by a fuel. If we know how many grams of water we heat up, and the change in the. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. A variety of fuels undergo combustion, and the products made can be identified. The fire triangle identifies the three things that are required for a fire to burn. Revision for caie chemistry igcse, including summary notes, exam questions by topic and videos for each module. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. 1 calorie is defined as the amount of heat that is required to increase the temperature of 1 g of water in 1°c. Calorimetry is used to measure amounts of heat transferred to or from a substance.
from www.linstitute.net
To do so, the heat is exchanged with a calibrated object. The fire triangle identifies the three things that are required for a fire to burn. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. 1 calorie is defined as the amount of heat that is required to increase the temperature of 1 g of water in 1°c. Revision for caie chemistry igcse, including summary notes, exam questions by topic and videos for each module. If we know how many grams of water we heat up, and the change in the. We can experimentally determine the relative amounts of energy released by a fuel. A variety of fuels undergo combustion, and the products made can be identified. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Calorimetry allows for the measurement of the amount of.
Edexcel IGCSE Chemistry 复习笔记 3.1.6 Practical Investigating Temperature
Calorimetry Igcse Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. If we know how many grams of water we heat up, and the change in the. Revision for caie chemistry igcse, including summary notes, exam questions by topic and videos for each module. A variety of fuels undergo combustion, and the products made can be identified. The fire triangle identifies the three things that are required for a fire to burn. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. 1 calorie is defined as the amount of heat that is required to increase the temperature of 1 g of water in 1°c. Calorimetry allows for the measurement of the amount of. We can experimentally determine the relative amounts of energy released by a fuel.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby Calorimetry Igcse Chemistry A variety of fuels undergo combustion, and the products made can be identified. To do so, the heat is exchanged with a calibrated object. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. If we know how many grams of water we heat up, and the change in the. The fire triangle identifies the three. Calorimetry Igcse Chemistry.
From www.savemyexams.co.uk
Practical Investigating Temperature Changes (3.1.6) Edexcel IGCSE Calorimetry Igcse Chemistry We can experimentally determine the relative amounts of energy released by a fuel. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. Calorimetry is used to measure amounts of heat transferred to or from a substance. 1 calorie is defined. Calorimetry Igcse Chemistry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Igcse Chemistry Revision for caie chemistry igcse, including summary notes, exam questions by topic and videos for each module. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. A variety of fuels undergo combustion, and the products made can be identified. 1 calorie is defined as the amount of heat that is required to increase the. Calorimetry Igcse Chemistry.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimetry Igcse Chemistry To do so, the heat is exchanged with a calibrated object. Revision for caie chemistry igcse, including summary notes, exam questions by topic and videos for each module. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. The fire triangle identifies the three things that are required for a fire to burn.. Calorimetry Igcse Chemistry.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Igcse Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. 1 calorie is defined as the amount of heat that is required to increase the temperature of 1 g of water in 1°c. The fire triangle identifies the three things that are required for a fire to burn. 4.11 describe simple calorimetry experiments for reactions such. Calorimetry Igcse Chemistry.
From chemistrytalk.org
Calorimetry ChemTalk Calorimetry Igcse Chemistry 1 calorie is defined as the amount of heat that is required to increase the temperature of 1 g of water in 1°c. Calorimetry allows for the measurement of the amount of. Calorimetry is used to measure amounts of heat transferred to or from a substance. The fire triangle identifies the three things that are required for a fire to. Calorimetry Igcse Chemistry.
From studylib.net
Calorimetry Worksheet Calorimetry Igcse Chemistry Revision for caie chemistry igcse, including summary notes, exam questions by topic and videos for each module. If we know how many grams of water we heat up, and the change in the. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. Calorimetry is used to measure amounts of heat transferred to. Calorimetry Igcse Chemistry.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry Calorimetry Igcse Chemistry 1 calorie is defined as the amount of heat that is required to increase the temperature of 1 g of water in 1°c. To do so, the heat is exchanged with a calibrated object. Calorimetry allows for the measurement of the amount of. Calorimetry is used to measure amounts of heat transferred to or from a substance. Revision for caie. Calorimetry Igcse Chemistry.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.1.6 Practical Investigating Temperature Calorimetry Igcse Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. The fire triangle identifies the three things that are required for a fire to burn. To do so, the heat is exchanged with a calibrated object. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. We can experimentally. Calorimetry Igcse Chemistry.
From www.pinterest.com.au
Pin on Science Friday Calorimetry Igcse Chemistry A variety of fuels undergo combustion, and the products made can be identified. Revision for caie chemistry igcse, including summary notes, exam questions by topic and videos for each module. The fire triangle identifies the three things that are required for a fire to burn. If we know how many grams of water we heat up, and the change in. Calorimetry Igcse Chemistry.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimetry Igcse Chemistry 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. The fire triangle identifies the three things that are required for a fire to burn. If we know how many grams of water we heat up, and the change in the. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Calorimetry Igcse Chemistry.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.1.2 Calorimetry翰林国际教育 Calorimetry Igcse Chemistry 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. We can experimentally determine the relative amounts of energy released by a fuel. A variety of fuels undergo combustion, and the products made can be identified. If we know how many grams of water we heat up, and the change in the. 4.11. Calorimetry Igcse Chemistry.
From www.youtube.com
Chemistry IGCSE Calorimetry YouTube Calorimetry Igcse Chemistry If we know how many grams of water we heat up, and the change in the. We can experimentally determine the relative amounts of energy released by a fuel. The fire triangle identifies the three things that are required for a fire to burn. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. 1 calorie. Calorimetry Igcse Chemistry.
From resource.studiaacademy.com
3.1 Energetics Studia Academy Resources Calorimetry Igcse Chemistry 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. A variety of fuels undergo combustion, and the products made can be identified. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. To. Calorimetry Igcse Chemistry.
From davida.davivienda.com
Specific Heat Calculations Worksheet Answers Printable Word Searches Calorimetry Igcse Chemistry A variety of fuels undergo combustion, and the products made can be identified. Calorimetry is used to measure amounts of heat transferred to or from a substance. The fire triangle identifies the three things that are required for a fire to burn. We can experimentally determine the relative amounts of energy released by a fuel. Revision for caie chemistry igcse,. Calorimetry Igcse Chemistry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Igcse Chemistry A variety of fuels undergo combustion, and the products made can be identified. If we know how many grams of water we heat up, and the change in the. Calorimetry allows for the measurement of the amount of. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. 3:02 describe simple calorimetry experiments. Calorimetry Igcse Chemistry.
From ar.inspiredpencil.com
Calorimeter Calorimetry Igcse Chemistry A variety of fuels undergo combustion, and the products made can be identified. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. 1. Calorimetry Igcse Chemistry.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Igcse Chemistry To do so, the heat is exchanged with a calibrated object. Revision for caie chemistry igcse, including summary notes, exam questions by topic and videos for each module. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. 1 calorie is defined as the amount of heat that is required to increase the temperature of. Calorimetry Igcse Chemistry.
From www.slideserve.com
PPT Purpose of the Experiment PowerPoint Presentation, free download Calorimetry Igcse Chemistry 1 calorie is defined as the amount of heat that is required to increase the temperature of 1 g of water in 1°c. The fire triangle identifies the three things that are required for a fire to burn. A variety of fuels undergo combustion, and the products made can be identified. 3.2 describe simple calorimetry experiments for reactions such as. Calorimetry Igcse Chemistry.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Igcse Chemistry 1 calorie is defined as the amount of heat that is required to increase the temperature of 1 g of water in 1°c. Calorimetry allows for the measurement of the amount of. We can experimentally determine the relative amounts of energy released by a fuel. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Calorimetry. Calorimetry Igcse Chemistry.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Igcse Chemistry Revision for caie chemistry igcse, including summary notes, exam questions by topic and videos for each module. 1 calorie is defined as the amount of heat that is required to increase the temperature of 1 g of water in 1°c. A variety of fuels undergo combustion, and the products made can be identified. If we know how many grams of. Calorimetry Igcse Chemistry.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:3.1.2 Calorimetry Calorimetry Igcse Chemistry We can experimentally determine the relative amounts of energy released by a fuel. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. If we know how many grams of water we heat up, and the change in the. Calorimetry allows for the measurement of the amount of. 1 calorie is defined as the amount. Calorimetry Igcse Chemistry.
From studylib.net
Calorimetry practice Calorimetry Igcse Chemistry To do so, the heat is exchanged with a calibrated object. 1 calorie is defined as the amount of heat that is required to increase the temperature of 1 g of water in 1°c. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. If we know how many grams of water we heat up, and. Calorimetry Igcse Chemistry.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimetry Igcse Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization salts dissolving in. 4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in. A variety of fuels undergo combustion, and the products made can be identified. If. Calorimetry Igcse Chemistry.