How Does Sulfuric Acid Speed Up Esterification . Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. As a result the reaction rate is increased. Concentrated sulfuric acid acts as a. The esterification is not the only thing catalysed by sulphuric acid. Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Increases reaction rate by lowering the activation energy. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen atom and. Basically, it’s catalytic activity is protonation of whatever. Concentrated sulfuric acid acts as a catalyst: Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Concentrated sulfuric acid is used as a catalyst, and has a dual role: Acts as a dehydrating agent, forcing the equilibrium.
from www.studocu.com
The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen atom and. The esterification is not the only thing catalysed by sulphuric acid. Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Acts as a dehydrating agent, forcing the equilibrium. As a result the reaction rate is increased. Concentrated sulfuric acid acts as a. Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Concentrated sulfuric acid acts as a catalyst: Increases reaction rate by lowering the activation energy. Basically, it’s catalytic activity is protonation of whatever.
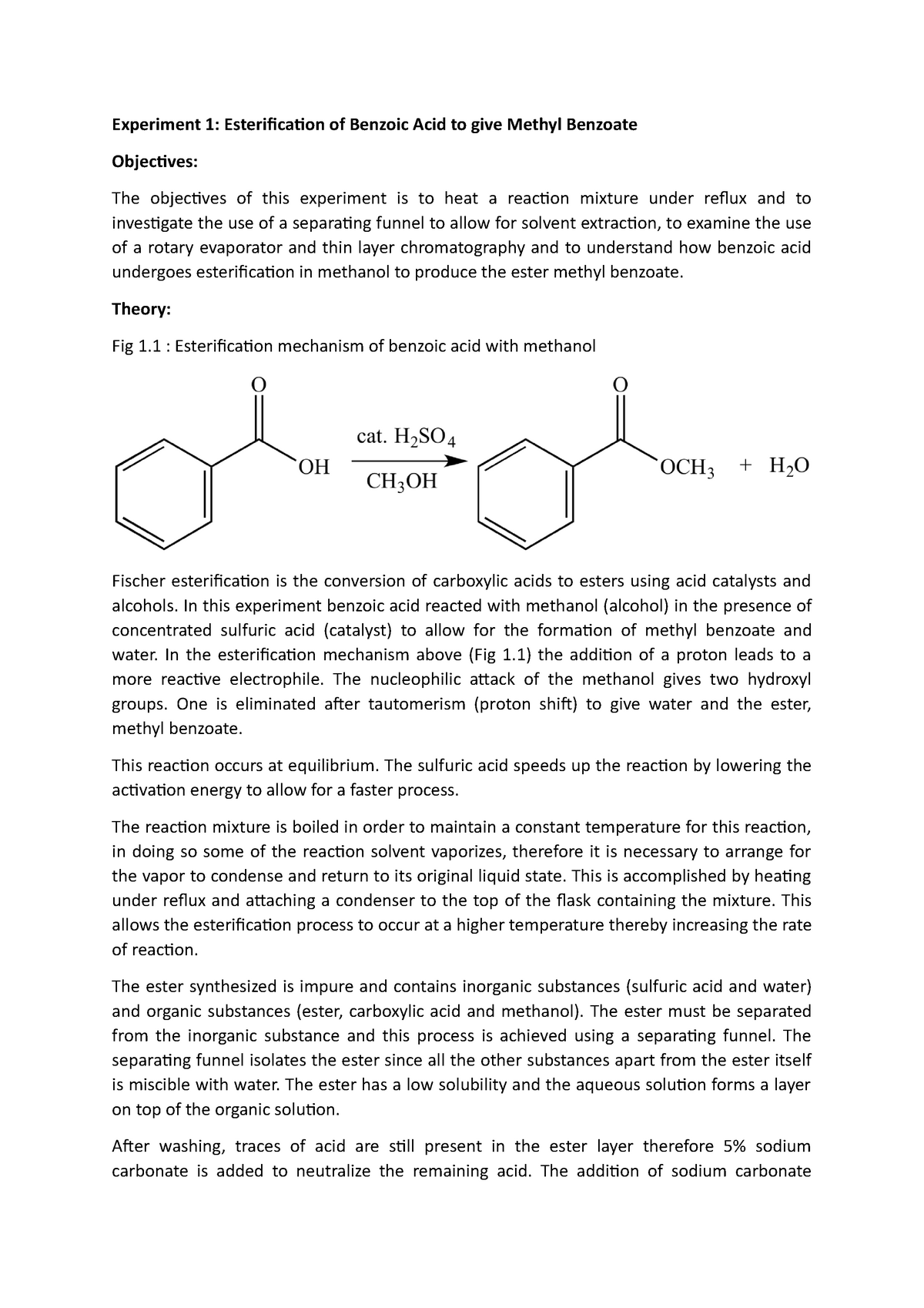
Esterification of benzoic acid to give methyl benzoate Theory Fig 1
How Does Sulfuric Acid Speed Up Esterification Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Concentrated sulfuric acid acts as a catalyst: Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Concentrated sulfuric acid is used as a catalyst, and has a dual role: The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen atom and. Basically, it’s catalytic activity is protonation of whatever. Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Increases reaction rate by lowering the activation energy. As a result the reaction rate is increased. Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; The esterification is not the only thing catalysed by sulphuric acid. Acts as a dehydrating agent, forcing the equilibrium. Concentrated sulfuric acid acts as a.
From www.youtube.com
Esterification Reaction of alcohol YouTube How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Basically, it’s catalytic activity is protonation of whatever. The esterification is not the only thing catalysed by sulphuric acid. Concentrated sulfuric acid acts as a. Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and.. How Does Sulfuric Acid Speed Up Esterification.
From www.masterorganicchemistry.com
Acid Catalysis of Organic Reactions Why It Works How Does Sulfuric Acid Speed Up Esterification As a result the reaction rate is increased. Basically, it’s catalytic activity is protonation of whatever. Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between. How Does Sulfuric Acid Speed Up Esterification.
From www.numerade.com
SOLVED Outline and then draw a reasonable reaction mechanism for the How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid acts as a catalyst: The esterification is not the only thing catalysed by sulphuric acid. Acts as a dehydrating agent, forcing the equilibrium. Concentrated sulfuric acid is used as a catalyst, and has a dual role: Basically, it’s catalytic activity is protonation of whatever. The π bond of the carbonyl group can act as a base to. How Does Sulfuric Acid Speed Up Esterification.
From orgosolver.com
OrgoSolver How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid acts as a catalyst: As a result the reaction rate is increased. Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Increases reaction rate. How Does Sulfuric Acid Speed Up Esterification.
From www.researchgate.net
Proposed reaction mechanism of stearic acid esterification over the How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid is used as a catalyst, and has a dual role: As a result the reaction rate is increased. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen atom and. Concentrated sulfuric acid is used as. How Does Sulfuric Acid Speed Up Esterification.
From pnghero.com
Sulfuric Acid Chemical Reaction Concentration Mechanism Esterification How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid is used as a catalyst, and has a dual role: Acts as a dehydrating agent, forcing the equilibrium. Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Concentrated sulfuric acid acts as a. Basically, it’s catalytic activity is protonation. How Does Sulfuric Acid Speed Up Esterification.
From animalia-life.club
Esterification Mechanism How Does Sulfuric Acid Speed Up Esterification Acts as a dehydrating agent, forcing the equilibrium. Basically, it’s catalytic activity is protonation of whatever. Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Increases reaction rate by lowering the activation energy. Concentrated sulfuric acid acts as a catalyst: Concentrated sulfuric. How Does Sulfuric Acid Speed Up Esterification.
From www.researchgate.net
(PDF) Study of Esterification of Free Fatty Acid in Low Grade How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Increases reaction rate by lowering the activation energy. Basically, it’s catalytic activity is protonation of whatever. Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Acts as a dehydrating agent, forcing the equilibrium. Concentrated. How Does Sulfuric Acid Speed Up Esterification.
From nrochemistry.com
Fischer Esterification How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid acts as a. Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen atom and. Basically, it’s catalytic activity is protonation. How Does Sulfuric Acid Speed Up Esterification.
From www.coursehero.com
[Solved] What is the purpose of adding sulfuric acid to an How Does Sulfuric Acid Speed Up Esterification Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Acts as a dehydrating agent, forcing the equilibrium. As a result the reaction rate is increased. Concentrated sulfuric acid is used as a catalyst, and has a dual role: Concentrated sulfuric acid acts. How Does Sulfuric Acid Speed Up Esterification.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic How Does Sulfuric Acid Speed Up Esterification The esterification is not the only thing catalysed by sulphuric acid. Concentrated sulfuric acid is used as a catalyst, and has a dual role: Concentrated sulfuric acid acts as a. Basically, it’s catalytic activity is protonation of whatever. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of. How Does Sulfuric Acid Speed Up Esterification.
From www.up.com
UP The Circular Economy of Sulfuric Acid How Does Sulfuric Acid Speed Up Esterification Acts as a dehydrating agent, forcing the equilibrium. Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Basically, it’s catalytic activity is protonation of whatever. As a result the reaction rate is increased. The esterification is not the only thing catalysed by sulphuric acid. Primarily, the benefit of sulfuric. How Does Sulfuric Acid Speed Up Esterification.
From www.researchgate.net
(PDF) of the esterification of phthalic anhydride with 2 How Does Sulfuric Acid Speed Up Esterification Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen atom and.. How Does Sulfuric Acid Speed Up Esterification.
From slideplayer.com
CONCLUSION to the BLUE LAB! ppt download How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Concentrated sulfuric acid is used as a catalyst, and has a dual role: Concentrated sulfuric acid acts as a. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion. How Does Sulfuric Acid Speed Up Esterification.
From www.youtube.com
Esterification Reaction Organic Chemistry YouTube How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid acts as a catalyst: Acts as a dehydrating agent, forcing the equilibrium. Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; As a result the reaction rate is increased. The π bond of the carbonyl group can act as. How Does Sulfuric Acid Speed Up Esterification.
From quizlet.com
What product is obtained from the reaction of sulfuric acid, Quizlet How Does Sulfuric Acid Speed Up Esterification Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; As a result the reaction rate is increased. Concentrated sulfuric acid acts as a catalyst: Concentrated sulfuric acid is used as a catalyst, and has a dual role: The π bond of the. How Does Sulfuric Acid Speed Up Esterification.
From www.researchgate.net
Tentative mechanisms of esterification between sulfuric acid and How Does Sulfuric Acid Speed Up Esterification Increases reaction rate by lowering the activation energy. Concentrated sulfuric acid acts as a. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen atom and. Acts as a dehydrating agent, forcing the equilibrium. Without this enhanced electrophilicity, the. How Does Sulfuric Acid Speed Up Esterification.
From blog.singaporestudent.com
b0039 esterification sulfuric acid How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid is used as a catalyst, and has a dual role: Concentrated sulfuric acid acts as a catalyst: Concentrated sulfuric acid acts as a. Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Primarily, the benefit of sulfuric acid in esterification is that it acts as a. How Does Sulfuric Acid Speed Up Esterification.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic How Does Sulfuric Acid Speed Up Esterification Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Concentrated sulfuric acid acts as a. Basically, it’s catalytic activity is protonation of whatever. The esterification is not the only thing catalysed by sulphuric acid. Concentrated sulfuric acid acts as a catalyst: Increases reaction rate by lowering the activation energy. Primarily, the benefit of sulfuric. How Does Sulfuric Acid Speed Up Esterification.
From www.masterorganicchemistry.com
Conversion of carboxylic acids to esters using acid and alcohols How Does Sulfuric Acid Speed Up Esterification The esterification is not the only thing catalysed by sulphuric acid. As a result the reaction rate is increased. Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; The π bond of the carbonyl group can act as a base to a. How Does Sulfuric Acid Speed Up Esterification.
From www.coursehero.com
[Solved] Write the mechanism for the Fischer esterification reaction of How Does Sulfuric Acid Speed Up Esterification Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Concentrated sulfuric acid acts as a catalyst: Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Basically, it’s catalytic activity is protonation of whatever. Concentrated sulfuric acid acts as a. Primarily, the benefit of. How Does Sulfuric Acid Speed Up Esterification.
From www.chegg.com
Solved What is the role of sulfuric acid in the reaction? How Does Sulfuric Acid Speed Up Esterification Acts as a dehydrating agent, forcing the equilibrium. Concentrated sulfuric acid acts as a catalyst: Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Increases reaction rate by lowering the activation energy. Basically, it’s catalytic activity is protonation of whatever. The esterification is not the only thing catalysed by sulphuric acid. The π bond. How Does Sulfuric Acid Speed Up Esterification.
From www.scribd.com
Argentina Esterification of Free Fatty Acids Using Sulfuric Acid As How Does Sulfuric Acid Speed Up Esterification Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Concentrated sulfuric acid acts as a. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen atom and. Basically, it’s catalytic activity is protonation. How Does Sulfuric Acid Speed Up Esterification.
From www.numerade.com
SOLVED Reaction Type Overall Reaction of 1butanol and acetic acid to How Does Sulfuric Acid Speed Up Esterification Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Increases reaction rate by lowering the activation energy. As a result the reaction rate is increased. Acts as a dehydrating agent, forcing the equilibrium. Concentrated sulfuric acid acts as a catalyst: Concentrated sulfuric acid is used as a catalyst, and has a dual role: Concentrated. How Does Sulfuric Acid Speed Up Esterification.
From www.researchgate.net
The reaction mechanism of sulfuric acid with polyethylene, introducing How Does Sulfuric Acid Speed Up Esterification Basically, it’s catalytic activity is protonation of whatever. Concentrated sulfuric acid acts as a catalyst: Concentrated sulfuric acid acts as a. Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Concentrated sulfuric acid is used as a catalyst, and has a dual role: Increases reaction rate by lowering the. How Does Sulfuric Acid Speed Up Esterification.
From docslib.org
Esterification of Ethanol and Acetic Acid in a Batch Reactor in How Does Sulfuric Acid Speed Up Esterification Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Concentrated sulfuric acid acts as a. Acts as a dehydrating agent, forcing the equilibrium. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen. How Does Sulfuric Acid Speed Up Esterification.
From www.dreamstime.com
The Manufacture of Sulphuric Acid Part1 Stock Vector Illustration of How Does Sulfuric Acid Speed Up Esterification Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Concentrated sulfuric acid is used as a catalyst, and has a dual role: The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen atom. How Does Sulfuric Acid Speed Up Esterification.
From www.wikidoc.org
Fischer esterification wikidoc How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Increases reaction rate by lowering the activation energy. Concentrated sulfuric acid is used. How Does Sulfuric Acid Speed Up Esterification.
From www.thestudentroom.co.uk
condensation polymerisation The Student Room How Does Sulfuric Acid Speed Up Esterification Acts as a dehydrating agent, forcing the equilibrium. Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Concentrated sulfuric acid acts as a catalyst: Concentrated sulfuric acid is used as a catalyst, and has a dual role: The esterification is not the. How Does Sulfuric Acid Speed Up Esterification.
From toolsweek.com
Does Sulfuric Acid Conduct Electricity? How Does Sulfuric Acid Speed Up Esterification The esterification is not the only thing catalysed by sulphuric acid. Increases reaction rate by lowering the activation energy. Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher,. How Does Sulfuric Acid Speed Up Esterification.
From eartheclipse.com
Does Sulfuric Acid Conduct Electricity? (Answered) Earth Eclipse How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Concentrated sulfuric acid acts as a catalyst: As a result the reaction rate is increased. The esterification is not the only thing catalysed by sulphuric acid. Concentrated sulfuric acid is used as a catalyst, and has a dual role: Without. How Does Sulfuric Acid Speed Up Esterification.
From www.studocu.com
Esterification of benzoic acid to give methyl benzoate Theory Fig 1 How Does Sulfuric Acid Speed Up Esterification Acts as a dehydrating agent, forcing the equilibrium. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference between the oxygen atom and. As a result the reaction rate is increased. Increases reaction rate by lowering the activation energy. Basically, it’s catalytic. How Does Sulfuric Acid Speed Up Esterification.
From www.coursehero.com
[Solved] Esterification Reactions Ethanol+ Ethanoic Acid + Sulfuric How Does Sulfuric Acid Speed Up Esterification Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6). Concentrated sulfuric acid acts as a. Acts as a dehydrating agent, forcing the. How Does Sulfuric Acid Speed Up Esterification.
From www.bartleby.com
Answered at purpose does the sulfuric acid play… bartleby How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid acts as a catalyst: Acts as a dehydrating agent, forcing the equilibrium. The esterification is not the only thing catalysed by sulphuric acid. Without this enhanced electrophilicity, the activation energy for nucleophilic attack would be much higher, and. Increases reaction rate by lowering the activation energy. As a result the reaction rate is increased. Basically, it’s catalytic. How Does Sulfuric Acid Speed Up Esterification.
From pdfprof.com
basic ester hydrolysis mechanism How Does Sulfuric Acid Speed Up Esterification Concentrated sulfuric acid is used as a catalyst, and has a dual role: Primarily, the benefit of sulfuric acid in esterification is that it acts as a proton donor, increasing the rate of reaction between the acid and the alcohol; Concentrated sulfuric acid is used as a catalyst to speed up the rate at which the ester is formed (6).. How Does Sulfuric Acid Speed Up Esterification.