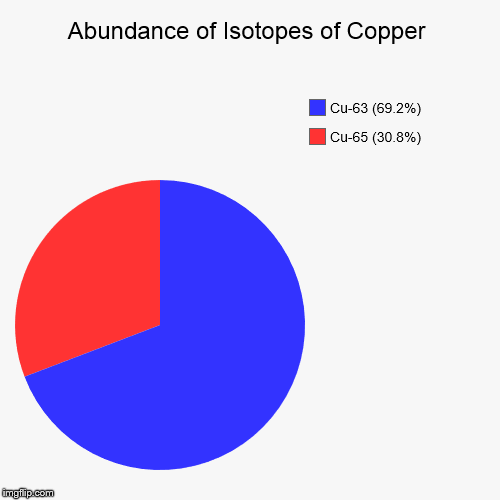
Copper Atomic Mass Isotopes . Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. For stable elements, there is usually a variety of stable isotopes. Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : Using a mass spectrometer, a scientist determined the percent abundances of the isotopes of sulfur to be 95.27% for 32 s, 0.51% for 33 s, and 4.22% for. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. These consist of an atomic nucleus with 29 protons and in the. All atomic nuclei of the chemical element copper are summarized under copper isotopes;
from imgflip.com
These consist of an atomic nucleus with 29 protons and in the. For stable elements, there is usually a variety of stable isotopes. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. All atomic nuclei of the chemical element copper are summarized under copper isotopes; Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Using a mass spectrometer, a scientist determined the percent abundances of the isotopes of sulfur to be 95.27% for 32 s, 0.51% for 33 s, and 4.22% for.
Copper Isotopic Abundance Imgflip
Copper Atomic Mass Isotopes All atomic nuclei of the chemical element copper are summarized under copper isotopes; Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Using a mass spectrometer, a scientist determined the percent abundances of the isotopes of sulfur to be 95.27% for 32 s, 0.51% for 33 s, and 4.22% for. All atomic nuclei of the chemical element copper are summarized under copper isotopes; This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. For stable elements, there is usually a variety of stable isotopes. Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. These consist of an atomic nucleus with 29 protons and in the.
From www.sciencephoto.com
Copper, atomic structure Stock Image C045/6369 Science Photo Library Copper Atomic Mass Isotopes 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Isotopes are nuclides that have the same atomic number and are. Copper Atomic Mass Isotopes.
From reviewhomedecor.co
Copper Periodic Table Protons And Neutrons Review Home Decor Copper Atomic Mass Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Using a mass spectrometer, a scientist determined. Copper Atomic Mass Isotopes.
From www.chegg.com
Solved 1. Estimate the atomic mass of copper. Copper has two Copper Atomic Mass Isotopes This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. All atomic nuclei of the chemical element copper are summarized under copper isotopes; For stable elements, there is usually a variety of stable isotopes. These consist of an atomic nucleus with 29 protons and in the. Copper (cu) atomic. Copper Atomic Mass Isotopes.
From material-properties.org
Copper Periodic Table and Atomic Properties Copper Atomic Mass Isotopes Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Sources, facts, uses, scarcity (sri), podcasts,. Copper Atomic Mass Isotopes.
From www.coursehero.com
[Solved] Copper has two naturally occurring isotopes. Cu63 has a mass Copper Atomic Mass Isotopes Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Copper (cu) atomic data for copper (cu). Copper Atomic Mass Isotopes.
From www.dreamstime.com
Copper Atom, with Mass and Energy Levels. Stock Vector Illustration Copper Atomic Mass Isotopes Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. For stable elements, there is usually a variety of stable isotopes. This table shows information about naturally occuring. Copper Atomic Mass Isotopes.
From www.hanlin.com
CIE A Level Chemistry复习笔记4.1.3 Isotopic Abundance & Relative Atomic Copper Atomic Mass Isotopes All atomic nuclei of the chemical element copper are summarized under copper isotopes; Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. For stable elements, there is usually a. Copper Atomic Mass Isotopes.
From murov.info
ATOMIC MASS and ISOTOPES Copper Atomic Mass Isotopes For stable elements, there is usually a variety of stable isotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. All atomic nuclei of the chemical element copper are. Copper Atomic Mass Isotopes.
From askfilo.com
Copper has two naturally occurring isotopes 63Cu and 65Cu. If the average.. Copper Atomic Mass Isotopes All atomic nuclei of the chemical element copper are summarized under copper isotopes; These consist of an atomic nucleus with 29 protons and in the. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. For stable elements, there is usually a. Copper Atomic Mass Isotopes.
From www.youtube.com
The atomic mass of copper is 63 546 amu Do any copper isotopes have a Copper Atomic Mass Isotopes Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. All atomic nuclei of the chemical element copper are summarized under copper isotopes; Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Using a mass spectrometer, a. Copper Atomic Mass Isotopes.
From brainly.com
Copper has two naturally occurring isotopes with masses 62.94 amu and Copper Atomic Mass Isotopes These consist of an atomic nucleus with 29 protons and in the. All atomic nuclei of the chemical element copper are summarized under copper isotopes; Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : For stable elements, there is usually a variety of stable isotopes. 60 rows copper (29 cu) has. Copper Atomic Mass Isotopes.
From www.toppr.com
The average atomic mass of copper is 63.546 amu. Natural copper Copper Atomic Mass Isotopes Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : For stable elements, there is usually a variety of stable isotopes. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average. Copper Atomic Mass Isotopes.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Copper Atomic Mass Isotopes This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : These consist of an atomic nucleus with 29 protons and in the. 60 rows copper (29 cu) has two stable isotopes,. Copper Atomic Mass Isotopes.
From periodictableguide.com
Copper (Cu) Periodic Table (Element Information & More) Copper Atomic Mass Isotopes All atomic nuclei of the chemical element copper are summarized under copper isotopes; Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : These consist of an atomic nucleus with 29 protons and in the. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. Copper Atomic Mass Isotopes.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Atomic Mass Isotopes All atomic nuclei of the chemical element copper are summarized under copper isotopes; 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. These consist of an atomic nucleus with 29 protons and in the. For stable elements, there is usually a variety of stable isotopes. This table shows information about naturally. Copper Atomic Mass Isotopes.
From www.pinterest.com
The Atom Chemistry Is My Jam! Atom, Chemistry notes, Chemistry help Copper Atomic Mass Isotopes Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : Using a mass spectrometer, a scientist determined the percent abundances. Copper Atomic Mass Isotopes.
From sanoberharald.blogspot.com
How To Work Out Relative Atomic Mass SanoberHarald Copper Atomic Mass Isotopes Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. These consist of an atomic nucleus with. Copper Atomic Mass Isotopes.
From www.numerade.com
⏩SOLVEDCalculate Copper has two isotopes C u63 (abundance =69.2 Copper Atomic Mass Isotopes All atomic nuclei of the chemical element copper are summarized under copper isotopes; Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Using a mass spectrometer, a scientist determined the percent abundances of the isotopes of sulfur to be 95.27% for 32 s, 0.51% for 33 s, and 4.22% for. For stable elements, there is usually a variety of. Copper Atomic Mass Isotopes.
From www.numerade.com
SOLVEDObtain the fractional abundances for the two naturally occurring Copper Atomic Mass Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. These consist of an atomic nucleus with 29 protons and in the. For stable elements, there is usually a variety of stable isotopes. Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546. Copper Atomic Mass Isotopes.
From present5.com
Isotopes Atoms of the same element with Copper Atomic Mass Isotopes Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the. Copper Atomic Mass Isotopes.
From chemistryismyjam.com
The Atom Chemistry Is My Jam! Copper Atomic Mass Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting. Copper Atomic Mass Isotopes.
From www.dreamstime.com
Carbon Isotopes. Atom Structure Stock Vector Illustration of molecule Copper Atomic Mass Isotopes 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. These consist of an atomic nucleus with 29 protons and in the. Using a mass spectrometer, a scientist determined the percent abundances of the isotopes of sulfur to be 95.27% for 32 s, 0.51% for 33 s, and 4.22% for. For stable. Copper Atomic Mass Isotopes.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID4825731 Copper Atomic Mass Isotopes This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. Copper (cu) atomic data for copper (cu). Copper Atomic Mass Isotopes.
From general.chemistrysteps.com
How To Calculate The Average Atomic Mass Chemistry Steps Copper Atomic Mass Isotopes Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. All atomic nuclei of the chemical element copper are summarized. Copper Atomic Mass Isotopes.
From www.numerade.com
SOLVEDNaturally occurring copper, Cu, is composed of two isotopes. The Copper Atomic Mass Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic. Copper Atomic Mass Isotopes.
From ecurrencythailand.com
What Is The Atomic Mass Of Copper 63 And Copper 65? All Answers Copper Atomic Mass Isotopes 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Copper (cu) atomic data for copper (cu) atomic number =. Copper Atomic Mass Isotopes.
From yoo.rs
What is the isotope? Yoors Copper Atomic Mass Isotopes This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : These consist of an atomic nucleus with 29 protons and in the. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos. Copper Atomic Mass Isotopes.
From www.nuclear-power.com
Iron Atomic Number Atomic Mass Density of Iron Copper Atomic Mass Isotopes This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. For stable elements, there is usually a variety of stable isotopes. These consist of an atomic nucleus with 29 protons and in the. Using a mass spectrometer, a scientist determined the percent abundances of the isotopes of sulfur to. Copper Atomic Mass Isotopes.
From www.numerade.com
SOLVED Copper has two isotopes, Cu63 and Cu65. The table below shows Copper Atomic Mass Isotopes These consist of an atomic nucleus with 29 protons and in the. All atomic nuclei of the chemical element copper are summarized under copper isotopes; This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu,. Copper Atomic Mass Isotopes.
From www.numerade.com
SOLVED Calculate Copper has two isotopes C u63 (abundance =69.2 Copper Atomic Mass Isotopes Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. For stable elements, there is usually a variety of stable isotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ. Copper Atomic Mass Isotopes.
From pubchem.ncbi.nlm.nih.gov
Copper Cu (Element) PubChem Copper Atomic Mass Isotopes Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : For stable elements, there is usually a variety of stable isotopes. This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and.. Copper Atomic Mass Isotopes.
From periodictable.me
Calculating+relative+atomic+mass Dynamic Periodic Table of Elements Copper Atomic Mass Isotopes 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. For stable elements, there is usually a variety of stable. Copper Atomic Mass Isotopes.
From www.numerade.com
Copper has an average atomic mass of 63.55 amu and two naturally Copper Atomic Mass Isotopes Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. These consist of an atomic nucleus with 29 protons and in the. 60 rows copper (29 cu) has two stable isotopes, 63 cu and 65 cu, along with 28 radioisotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number. Copper Atomic Mass Isotopes.
From periodictable.me
Way to Find Atomic Mass of Elements Dynamic Periodic Table of Copper Atomic Mass Isotopes This table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins, and their magnetic moments. Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Copper Atomic Mass Isotopes.
From imgflip.com
Copper Isotopic Abundance Imgflip Copper Atomic Mass Isotopes Copper has two isotopes, 63 cu (69.15%, mass=62.9300 amu) and 65 cu (30.85%, mass = 64.928 amu), and so the respective mole fractions are 0.6915 and 0.3085, resulting in an average atomic. Copper (cu) atomic data for copper (cu) atomic number = 29 atomic weight = 63.546 reference e95 : Using a mass spectrometer, a scientist determined the percent abundances. Copper Atomic Mass Isotopes.