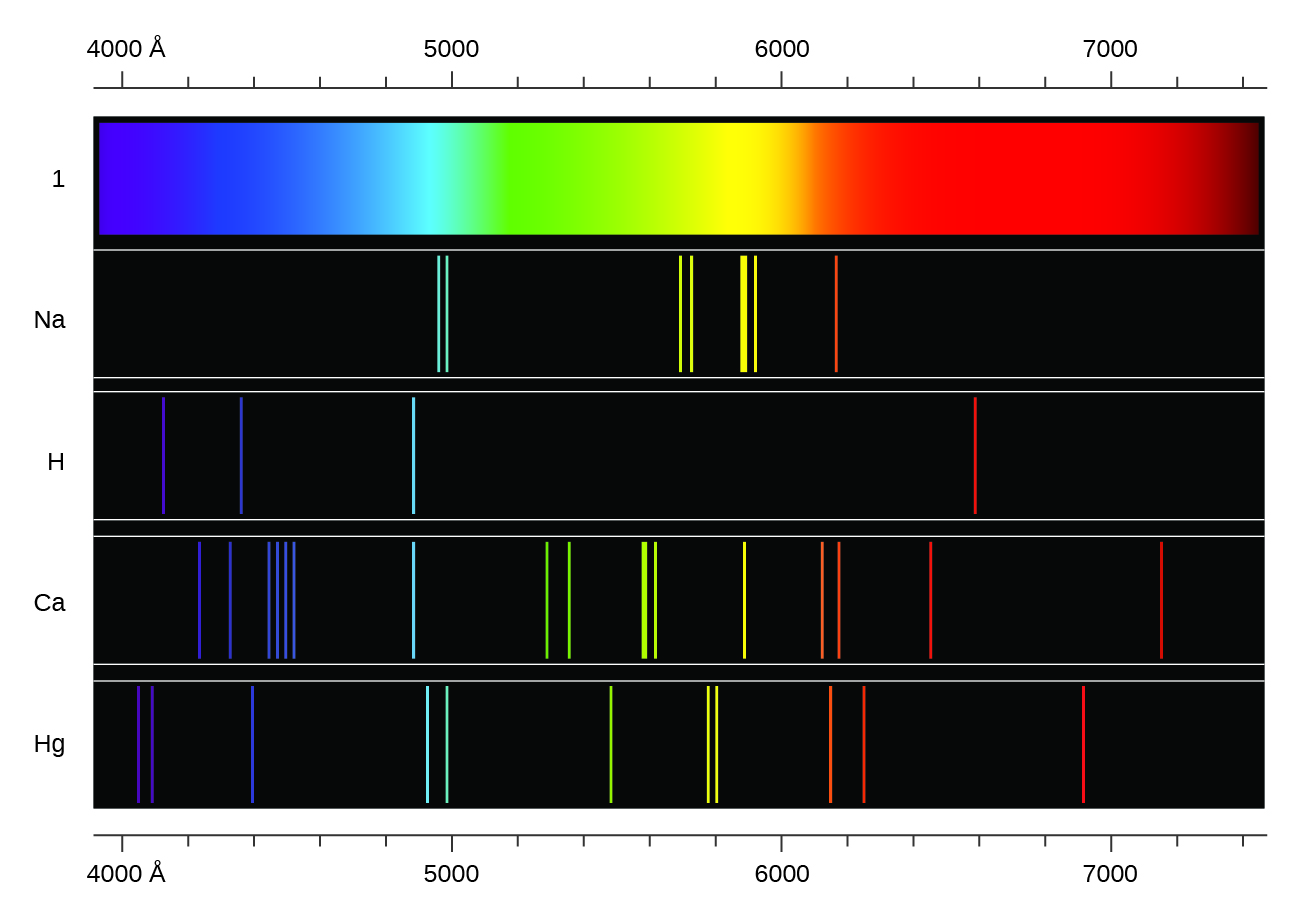
Emission Spectra Table . Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. The emission spectra of various atoms. (if ti ≠ te) format output: The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet order. All only with transition probabilities only with energy level. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. The ground state of an atom is the lowest energy state.
from wisc.pb.unizin.org
Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. (if ti ≠ te) format output: All only with transition probabilities only with energy level. The emission spectra of various atoms. The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet order. The ground state of an atom is the lowest energy state.
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/
Emission Spectra Table (if ti ≠ te) format output: Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. The emission spectra of various atoms. The ground state of an atom is the lowest energy state. All only with transition probabilities only with energy level. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet order. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. (if ti ≠ te) format output:
From www.umop.net
Visible Spectra of the Elements Emission Spectra Table The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. The ground state of an atom is the lowest energy state. (if ti ≠ te) format output:. Emission Spectra Table.
From askfilo.com
SECTION AQ. 1 Select and write the correct answeri) How many grams o.. Emission Spectra Table (if ti ≠ te) format output: The emission spectra of various atoms. All only with transition probabilities only with energy level. Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet order. Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted. Emission Spectra Table.
From umop.net
Visible Spectra of the Elements Emission Spectra Table (if ti ≠ te) format output: Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. Since each atom has many excited states (high energy. Emission Spectra Table.
From www.periodictableprintable.com
Periodic Table Atomic Emission Spectra 2024 Periodic Table Printable Emission Spectra Table The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. (if ti ≠ te) format output: Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. The emission spectrum (or line spectrum) of. Emission Spectra Table.
From www.reddit.com
Emission Spectra of the Elements in Periodic Table Format r/chemistry Emission Spectra Table All only with transition probabilities only with energy level. (if ti ≠ te) format output: The emission spectra of various atoms. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. The ground state of an atom is the lowest energy state. Since each atom has many excited states (high energy levels). Emission Spectra Table.
From www.degruyter.com
Spinbearing molecules as optically addressable platforms for quantum Emission Spectra Table Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. (if ti ≠ te) format output: The emission spectra of various atoms. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low. Emission Spectra Table.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Emission Spectra Table Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet order. The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. The ground state of an atom is the lowest energy state. All only with transition probabilities only. Emission Spectra Table.
From www.aanda.org
OH midinfrared emission as a diagnostic of H2O UV photodissociation Emission Spectra Table Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. The ground state of an atom is the lowest energy state. All only with transition probabilities only with energy level. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that. Emission Spectra Table.
From www.pinterest.com
line spectra chart If the emission lines of the chemical elements Emission Spectra Table Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Since each atom has many excited states (high. Emission Spectra Table.
From www.toppr.com
Define emission spectron and absorption spectrum. Emission Spectra Table The ground state of an atom is the lowest energy state. Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. (if ti ≠ te) format output: All only with transition probabilities only with energy level. The emission spectrum can be used to determine the composition of. Emission Spectra Table.
From www.reddit.com
Periodic table of each elements emission spectrum with regular periodic Emission Spectra Table The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. All only with transition probabilities only with energy level. The ground. Emission Spectra Table.
From www.chegg.com
uld see that every single one of them would put out Emission Spectra Table Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet order. Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the. Emission Spectra Table.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Table The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. (if ti ≠ te) format output: All only. Emission Spectra Table.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Spectra Table Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. The ground state of an atom is the lowest energy state. (if ti ≠ te) format output: The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light. Emission Spectra Table.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra Table (if ti ≠ te) format output: The ground state of an atom is the lowest energy state. Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of. Emission Spectra Table.
From uwaterloo.ca
Periodic Table of emission spectra Chem 13 News Magazine University Emission Spectra Table New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. (if ti ≠ te) format output: The ground state of an atom is the lowest energy state.. Emission Spectra Table.
From digital-library.theiet.org
Radar emission spectrum engineering Novel Radar Techniques and Emission Spectra Table Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. The emission spectrum can be used to determine the composition of a. Emission Spectra Table.
From www.carolina.com
Periodic Table of Spectra, Poster Carolina Biological Supply Emission Spectra Table (if ti ≠ te) format output: Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. All only with transition probabilities only with energy level.. Emission Spectra Table.
From www.pinterest.com
SHAPES OF ATOMIC ORBITALS Physics and mathematics, Teaching chemistry Emission Spectra Table Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the. Emission Spectra Table.
From alquilercastilloshinchables.info
8 Pics Ir Spectrum Table Of Compounds And Review Alqu Blog Emission Spectra Table All only with transition probabilities only with energy level. The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible.. Emission Spectra Table.
From pmc.ncbi.nlm.nih.gov
A highly fluorescent bora[6]helicene exhibiting circularly polarized Emission Spectra Table The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. The emission spectrum can be used to determine the composition of a material, since it is different. Emission Spectra Table.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Emission Spectra Table Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet order. The emission spectra of various atoms. The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. The emission spectrum (or line spectrum) of a chemical element is. Emission Spectra Table.
From whitsonmysecutage.blogspot.com
Continuous Spectrum Vs Emission Spectrum Vs Absorption Spectrum Emission Spectra Table The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. Since each atom has many excited states (high energy levels) available to it, several colors of light. Emission Spectra Table.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Table All only with transition probabilities only with energy level. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as. Emission Spectra Table.
From joihsprvv.blob.core.windows.net
Emission Spectra Bbc Bitesize at Jose Doty blog Emission Spectra Table The emission spectra of various atoms. All only with transition probabilities only with energy level. (if ti ≠ te) format output: New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels and. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the. Emission Spectra Table.
From www.semanticscholar.org
Figure 1 from A stratified jet model for AGN emission in the twoflow Emission Spectra Table The ground state of an atom is the lowest energy state. Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as. Emission Spectra Table.
From brokeasshome.com
periodic table emission spectra Emission Spectra Table Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. All only with transition probabilities only with energy level. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. (if. Emission Spectra Table.
From chemwiki.ucdavis.edu
The Bohr Atom Chemwiki Emission Spectra Table Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. (if ti ≠ te) format output: All only with transition probabilities only with energy level. The ground state of an atom is the lowest energy state. The emission spectrum (or line spectrum) of a chemical element is. Emission Spectra Table.
From chem.libretexts.org
Chapter 2.3 Atomic Spectra and Models of the Atom Chemistry LibreTexts Emission Spectra Table The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Atomic emission spectra the electrons in an atom tend to be. Emission Spectra Table.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Emission Spectra Table The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. The emission spectra of various atoms. The ground state of an atom is the lowest energy state. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the. Emission Spectra Table.
From www.pinterest.com
Pin on AP Chem 5 Atomic Structure and Periodicity Emission Spectra Table Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet order. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. (if ti ≠ te) format output: The emission spectrum can be used to determine the composition. Emission Spectra Table.
From jamshimi.ir
روش های تولید و ایجاد طیف نشری و طیف خطی عنصر ها و تفاوت آن ها جم شیمی Emission Spectra Table Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet order. Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. The emission spectrum (or line spectrum) of a chemical element is the unique pattern. Emission Spectra Table.
From chemwiki.ucdavis.edu
Light, Particles, and Waves Chemwiki Emission Spectra Table The emission spectra of various atoms. All only with transition probabilities only with energy level. Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. The emission spectrum can be used to determine the composition of a material, since it is different for each element of the. Emission Spectra Table.
From poplitizscstudyquizz.z13.web.core.windows.net
Isotopes And Ions Worksheet Key Emission Spectra Table Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet order. The emission spectrum can be used to determine the composition of a material, since it is different for each element of the periodic table. New graphical features include dynamic transition plots (grotrian diagrams) with ability to zoom into particular energy levels. Emission Spectra Table.
From patents.google.com
WO2024180566A1 Polymerizable luminescent solventfree organic liquids Emission Spectra Table Atomic emission spectra the electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. The emission spectrum can be used to determine the composition. Emission Spectra Table.