Equipment Validation Meaning . Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. They each have a different role to play. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. As part of process validation through iq/oq/pq, laboratories. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications.
from pharmaguddu.com
An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. As part of process validation through iq/oq/pq, laboratories. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. They each have a different role to play.
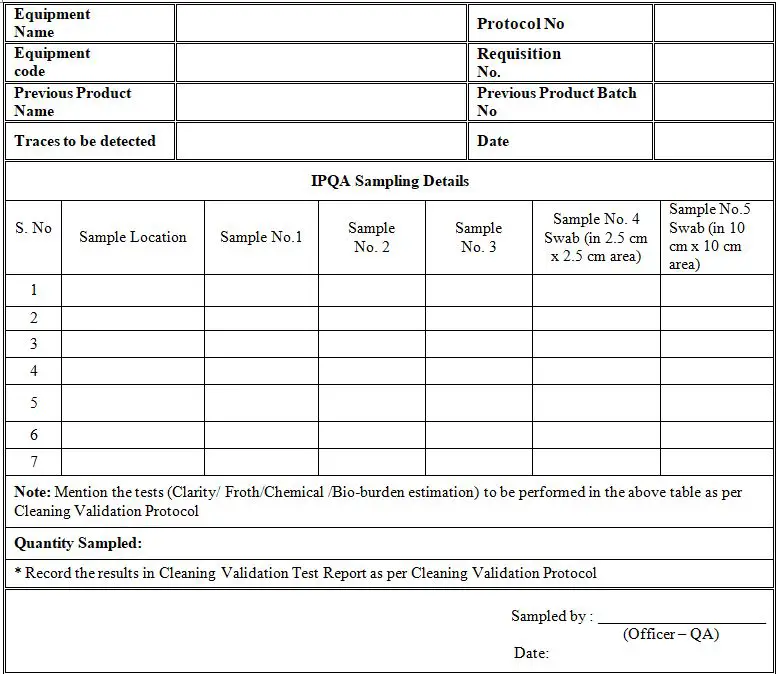
Cleaning Validation Protocol for Pharmaceutical Equipments » Pharmaguddu
Equipment Validation Meaning An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. As part of process validation through iq/oq/pq, laboratories. They each have a different role to play. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory.
From pharmaguddu.com
Cleaning Validation Protocol for Pharmaceutical Equipments » Pharmaguddu Equipment Validation Meaning As part of process validation through iq/oq/pq, laboratories. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Iq, oq and pq are the three steps of process validation that regulators such as the. Equipment Validation Meaning.
From www.qualitymeddev.com
Design Verification vs Design Validation What are The Differences Equipment Validation Meaning Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or. Equipment Validation Meaning.
From www.presentationeze.com
test equipment validation PresentationEZE Equipment Validation Meaning Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. As part of process validation through iq/oq/pq, laboratories. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is the process. Equipment Validation Meaning.
From fivevalidation.com
Verification & Validation V&V FIVE Validation Equipment Validation Meaning They each have a different role to play. Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. As part of process validation through iq/oq/pq, laboratories. Equipment validation is a term used to describe a set of independent procedures that are used. Equipment Validation Meaning.
From www.presentationeze.com
Product and Process Validation Full Details PresentationEZE Equipment Validation Meaning As part of process validation through iq/oq/pq, laboratories. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. Equipment validation is. Equipment Validation Meaning.
From www.slideserve.com
PPT 3. Validation (and Qualification) PowerPoint Presentation, free Equipment Validation Meaning Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. They each have a different role to play. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various. Equipment Validation Meaning.
From www.slideshare.net
Validation of equipment copy Equipment Validation Meaning Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Ensuring laboratory equipment operates as expected is critically important to the validity of. Equipment Validation Meaning.
From www.slideshare.net
Validation of equipment copy Equipment Validation Meaning Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. They each have a different role to play. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. Equipment validation is. Equipment Validation Meaning.
From blog.sierralabs.com
The Secret to GxP Cloud Compliance in 4 Minutes or Less Equipment Validation Meaning Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines. Equipment Validation Meaning.
From www.presentationeze.com
Equipment Validation FAT, DQ, IQ, OQ, PQ, URS.PresentationEZE Equipment Validation Meaning Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. As part of process validation through iq/oq/pq, laboratories. Equipment validation is the process of validating the requirements,. Equipment Validation Meaning.
From www.browserstack.com
Verification and Validation in Software Testing BrowserStack Equipment Validation Meaning Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety. Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. Equipment validation. Equipment Validation Meaning.
From www.slideserve.com
PPT Validation Part 5 Review and summary PowerPoint Presentation Equipment Validation Meaning Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. As part of process validation through iq/oq/pq, laboratories. Equipment validation is a systematic and documented process that verifies that equipment, such. Equipment Validation Meaning.
From www.slideshare.net
Validation of equipments Equipment Validation Meaning They each have a different role to play. Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. As part of process validation through iq/oq/pq, laboratories. Iq, oq and pq are the three steps of process validation that regulators such as the fda require you. Equipment Validation Meaning.
From kvalito.ch
How is a system validated? Kvalito Equipment Validation Meaning Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping,. Equipment Validation Meaning.
From www.slideshare.net
EQUIPMENT VALIDATION Equipment Validation Meaning As part of process validation through iq/oq/pq, laboratories. They each have a different role to play. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment,. Equipment Validation Meaning.
From mac51.blogspot.com
What are 4 must know Types of Validations in pharmaceutical industry FDA Equipment Validation Meaning An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. Ensuring laboratory equipment operates as expected. Equipment Validation Meaning.
From www.slideshare.net
Validation of equipments Equipment Validation Meaning Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. Equipment validation is the process of validating the requirements, specifications, and uses of a. Equipment Validation Meaning.
From pharmaguddu.com
Difference Between Validation, Calibration, and Qualification in Pharma Equipment Validation Meaning As part of process validation through iq/oq/pq, laboratories. They each have a different role to play. Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of. Equipment Validation Meaning.
From joijmacyp.blob.core.windows.net
Laboratory Data Validation at John Childress blog Equipment Validation Meaning Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. As part of process validation through iq/oq/pq, laboratories. Iq, oq and pq are the. Equipment Validation Meaning.
From www.scribd.com
Validation of Equipment Verification And Validation Specification Equipment Validation Meaning Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety. Ensuring laboratory equipment operates as expected is critically important to the validity. Equipment Validation Meaning.
From www.getreskilled.com
Qualification vs Validation in Pharma GetReskilled Equipment Validation Meaning Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. As part of process validation through iq/oq/pq, laboratories. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that. Equipment Validation Meaning.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Equipment Validation Meaning They each have a different role to play. Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as. Equipment Validation Meaning.
From www.studocu.com
Chapter 3 Equipment Validation Equipment Validation Objective At Equipment Validation Meaning Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is a documented process demonstrating whether a piece of equipment, system,. Equipment Validation Meaning.
From www.slideserve.com
PPT Validation of Pharmaceutical Packaging PowerPoint Presentation Equipment Validation Meaning Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment. Equipment Validation Meaning.
From pharmdguru.com
3. VALIDATION METHODS QUALITY OF EQUIPMENT, VALIDATION OF EQUIPMENT Equipment Validation Meaning Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is. Equipment Validation Meaning.
From www.tpsearchtool.com
Iq Oq And Pq Validation Services Gk Bioscience Images Equipment Validation Meaning Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent. Equipment Validation Meaning.
From www.slideshare.net
Validation of equipments Equipment Validation Meaning Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. As part of process validation through iq/oq/pq, laboratories. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. They each have a different. Equipment Validation Meaning.
From www.presentationeze.com
Equipment Validation Facility Qualification Material Equipment Validation Meaning Iq, oq and pq are the three steps of process validation that regulators such as the fda require you to develop to ensure consistent outputs from your equipment. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is. Equipment Validation Meaning.
From mudassiriqbal.net
What is Data Validation and Why It Is Necessary Mudassir Iqbal Equipment Validation Meaning An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. Equipment validation is a documented process. Equipment Validation Meaning.
From www.presentationeze.com
Equipment Validation PresentationEZE Equipment Validation Meaning Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Iq, oq and pq are the three steps of process validation that regulators. Equipment Validation Meaning.
From dokumen.tips
(PPT) Validation of equipments DOKUMEN.TIPS Equipment Validation Meaning Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety. An equipment validation protocol (also known as an equipment qualification protocol) is. Equipment Validation Meaning.
From www.youtube.com
Validation — what is VALIDATION meaning YouTube Equipment Validation Meaning They each have a different role to play. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. Equipment validation. Equipment Validation Meaning.
From www.slideshare.net
Validation, scope of validation, URS , WHO GUIDELINES FOR VALIDATION Equipment Validation Meaning Equipment validation is a term used to describe a set of independent procedures that are used to check if a product meets the specifications and. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. Ensuring laboratory equipment operates as expected is critically important to the validity of results. Equipment Validation Meaning.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Equipment Validation Meaning Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Equipment validation is a documented process demonstrating whether a piece of equipment, system, or process consistently produces results within predefined specifications. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. An equipment validation. Equipment Validation Meaning.
From www.pharmaqualification.com
Prospective Process Validation Equipment Validation Meaning Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety. Equipment validation is a term used to describe a set of independent. Equipment Validation Meaning.