Catalysis Organic Chemistry Reaction . In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. This perspective aims to introduce the different categories of dual catalytic systems and demonstrate their benefits in constructing new chemical bonds and enhanced stereoselectivity. This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the remarkable recent impact of this area on the field of organic reaction invention and its application in both industrial and academic settings. Free carbenes are transient reactive intermediates in a broad range of organic transformations. In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant.
from www.masterorganicchemistry.com
This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. This perspective aims to introduce the different categories of dual catalytic systems and demonstrate their benefits in constructing new chemical bonds and enhanced stereoselectivity. First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the remarkable recent impact of this area on the field of organic reaction invention and its application in both industrial and academic settings. In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. Free carbenes are transient reactive intermediates in a broad range of organic transformations. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes.
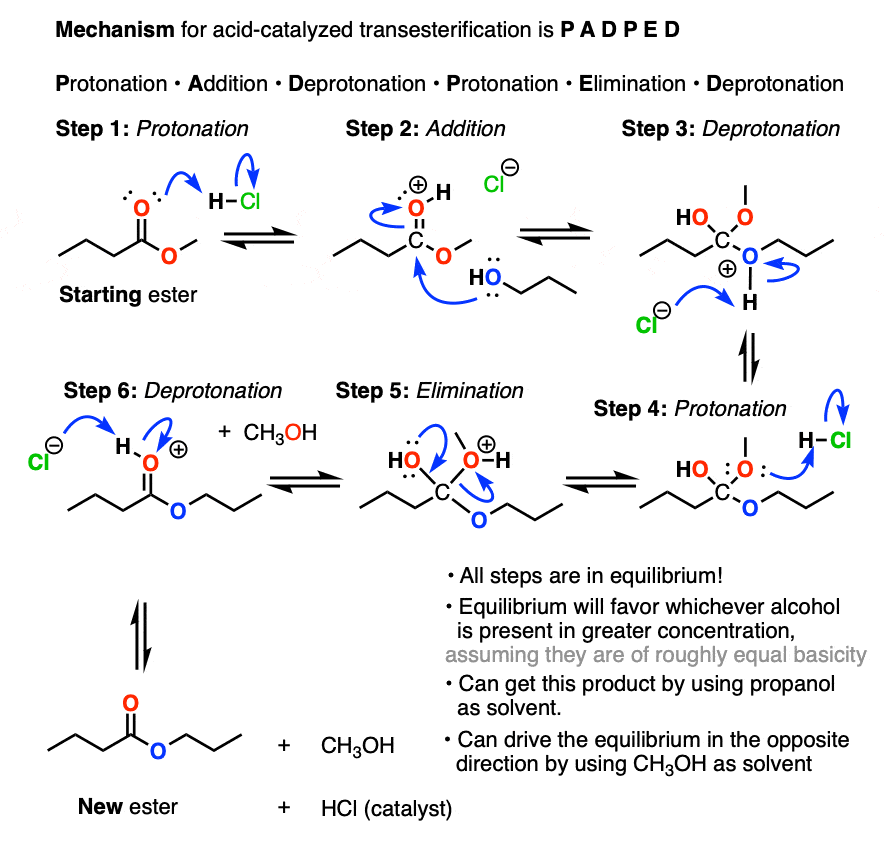
Transesterification Master Organic Chemistry
Catalysis Organic Chemistry Reaction First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the remarkable recent impact of this area on the field of organic reaction invention and its application in both industrial and academic settings. In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. This perspective aims to introduce the different categories of dual catalytic systems and demonstrate their benefits in constructing new chemical bonds and enhanced stereoselectivity. In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. Free carbenes are transient reactive intermediates in a broad range of organic transformations. First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the remarkable recent impact of this area on the field of organic reaction invention and its application in both industrial and academic settings. This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes.
From www.researchgate.net
Catalysis reactions of some organic components by metal nanoparticles Catalysis Organic Chemistry Reaction A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. This perspective aims to introduce the different categories of dual catalytic systems and demonstrate their benefits in constructing new chemical bonds and enhanced stereoselectivity. Dual catalysis is one of the most powerful strategies for the development of. Catalysis Organic Chemistry Reaction.
From www.bol.com
Iron Catalysis in Organic Chemistry Reactions and Applications Catalysis Organic Chemistry Reaction A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. Free carbenes are transient reactive intermediates in a broad range of organic transformations. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. This overview is arranged by catalyst. Catalysis Organic Chemistry Reaction.
From www.masterorganicchemistry.com
Transesterification Master Organic Chemistry Catalysis Organic Chemistry Reaction Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. Free carbenes are transient reactive intermediates in a broad range of. Catalysis Organic Chemistry Reaction.
From www.youtube.com
01.10 Base Catalysis and Summary YouTube Catalysis Organic Chemistry Reaction These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. This perspective aims to introduce the different categories of dual catalytic systems and demonstrate their benefits in constructing new chemical bonds and enhanced stereoselectivity. In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. Free carbenes are transient reactive. Catalysis Organic Chemistry Reaction.
From www.taylorfrancis.com
Catalysis of Organic Reactions Taylor & Francis Group Catalysis Organic Chemistry Reaction A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the remarkable recent impact of this area on the field of organic reaction invention and its application in both. Catalysis Organic Chemistry Reaction.
From www.researchgate.net
Mechanisms of ester hydrolysis under acid or base catalysis. Download Catalysis Organic Chemistry Reaction In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. Free carbenes. Catalysis Organic Chemistry Reaction.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID4409461 Catalysis Organic Chemistry Reaction This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. This. Catalysis Organic Chemistry Reaction.
From www.taylorfrancis.com
Catalysis of Organic Reactions Taylor & Francis Group Catalysis Organic Chemistry Reaction In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not. Catalysis Organic Chemistry Reaction.
From slidetodoc.com
Chapter 23 Catalysis in Organic Reactions and in Catalysis Organic Chemistry Reaction A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the. Catalysis Organic Chemistry Reaction.
From www.science.org
Merging Photoredox Catalysis with Organocatalysis The Direct Catalysis Organic Chemistry Reaction Free carbenes are transient reactive intermediates in a broad range of organic transformations. This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a. Catalysis Organic Chemistry Reaction.
From slidetodoc.com
Chapter 23 Catalysis in Organic Reactions and in Catalysis Organic Chemistry Reaction In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. Free carbenes are transient reactive intermediates in a broad range of organic transformations. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is. Catalysis Organic Chemistry Reaction.
From quizlet.com
Organic Chemistry Reaction Pathways Diagram Quizlet Catalysis Organic Chemistry Reaction In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. In. Catalysis Organic Chemistry Reaction.
From pubs.acs.org
Photoredox Catalysis in Organic Chemistry The Journal of Organic Catalysis Organic Chemistry Reaction This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. Free carbenes are transient reactive intermediates in a broad range of organic transformations. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not. Catalysis Organic Chemistry Reaction.
From www.scribd.com
Catalysis( From Royal Society of Chemistry) Catalysis Chemical Catalysis Organic Chemistry Reaction First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the remarkable recent impact of this area on the field of organic reaction invention and its application in both industrial and academic settings. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. Free. Catalysis Organic Chemistry Reaction.
From www.masterorganicchemistry.com
Alcohols To Ethers via Acid Catalysis Master Organic Chemistry Catalysis Organic Chemistry Reaction This perspective aims to introduce the different categories of dual catalytic systems and demonstrate their benefits in constructing new chemical bonds and enhanced stereoselectivity. Free carbenes are transient reactive intermediates in a broad range of organic transformations. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. First, we will examine the. Catalysis Organic Chemistry Reaction.
From www.researchgate.net
Use of heterogeneous catalysis in various chemistry sectors Download Catalysis Organic Chemistry Reaction A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. Free carbenes are transient reactive intermediates in a broad range of organic transformations. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. In contrast, the use of heterogeneous. Catalysis Organic Chemistry Reaction.
From www.slideserve.com
PPT Surfactants as Catalysts for Organic Reactions in Water Catalysis Organic Chemistry Reaction In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. In contrast,. Catalysis Organic Chemistry Reaction.
From slidetodoc.com
Chapter 23 Catalysis in Organic Reactions and in Catalysis Organic Chemistry Reaction These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. A catalyst increases the rate of a. Catalysis Organic Chemistry Reaction.
From www.slideserve.com
PPT Surfactants as Catalysts for Organic Reactions in Water Catalysis Organic Chemistry Reaction This perspective aims to introduce the different categories of dual catalytic systems and demonstrate their benefits in constructing new chemical bonds and enhanced stereoselectivity. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. Free carbenes are transient reactive intermediates in a broad range of organic transformations. This overview is arranged by. Catalysis Organic Chemistry Reaction.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Catalysis Organic Chemistry Reaction In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. First, we will examine the utility of photoredox catalysis from. Catalysis Organic Chemistry Reaction.
From www.masterorganicchemistry.com
Acid Catalysis of Organic Reactions Why It Works Catalysis Organic Chemistry Reaction In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. Free carbenes are transient reactive intermediates in a broad range of organic transformations. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. This perspective aims to introduce the different categories of dual catalytic systems and demonstrate their benefits. Catalysis Organic Chemistry Reaction.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysis Organic Chemistry Reaction In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. Free carbenes are transient reactive intermediates in a broad range of organic transformations. First, we will examine the utility of. Catalysis Organic Chemistry Reaction.
From www.masterorganicchemistry.com
Enol Reactions AcidCatalyzed Aldol, Halogenation, and Mannich Reaction Catalysis Organic Chemistry Reaction A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. In this review, we highlight the. Catalysis Organic Chemistry Reaction.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Catalysis Organic Chemistry Reaction First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the remarkable recent impact of this area on the field of organic reaction invention and its application in both industrial and academic settings. In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. This perspective aims to introduce the. Catalysis Organic Chemistry Reaction.
From 2012books.lardbucket.org
Catalysis Catalysis Organic Chemistry Reaction Free carbenes are transient reactive intermediates in a broad range of organic transformations. In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. Dual catalysis is one of the most powerful strategies for the development of chemical reactions in organic synthesis. This overview is arranged by catalyst class. Catalysis Organic Chemistry Reaction.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalysis Organic Chemistry Reaction A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. Dual catalysis is one of the most powerful strategies. Catalysis Organic Chemistry Reaction.
From slidetodoc.com
Chapter 23 Catalysis in Organic Reactions and in Catalysis Organic Chemistry Reaction First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the remarkable recent impact of this area on the field of organic reaction invention and its application in both industrial and academic settings. This perspective aims to introduce the different categories of dual catalytic systems and demonstrate their benefits in constructing new. Catalysis Organic Chemistry Reaction.
From www.slideserve.com
PPT Surfactants as Catalysts for Organic Reactions in Water Catalysis Organic Chemistry Reaction This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. This perspective aims to introduce the different categories of dual catalytic systems and demonstrate their benefits in constructing new chemical bonds and enhanced stereoselectivity. Dual catalysis is one of the most powerful. Catalysis Organic Chemistry Reaction.
From www.slideserve.com
PPT Classification of Catalyst Systems PowerPoint Presentation, free Catalysis Organic Chemistry Reaction In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. Free carbenes are transient reactive intermediates. Catalysis Organic Chemistry Reaction.
From pubs.acs.org
Photoredox Catalysis in Organic Chemistry The Journal of Organic Catalysis Organic Chemistry Reaction In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. Free carbenes are transient reactive intermediates in a broad range of organic transformations. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. In contrast,. Catalysis Organic Chemistry Reaction.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Catalysis Organic Chemistry Reaction First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the remarkable recent impact of this area on the field of organic reaction invention and its application in both industrial and academic settings. In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a. Catalysis Organic Chemistry Reaction.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Catalysis Organic Chemistry Reaction In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. A catalyst increases the rate of a reaction, but does not get consumed in the reaction and does not alter the equilibrium constant. These materials bridge the gap between organometallic and nanoparticle catalysis and are opening exciting avenues for mimicking metalloenzymes. This overview is arranged by catalyst. Catalysis Organic Chemistry Reaction.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalysis Organic Chemistry Reaction In this review, we highlight the use of organic photoredox catalysts in a myriad of synthetic transformations with a range of applications. First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the remarkable recent impact of this area on the field of organic reaction invention and its application in both industrial. Catalysis Organic Chemistry Reaction.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysis Organic Chemistry Reaction In contrast, the use of heterogeneous catalysts facilitates catalyst recycling by allowing simple. This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. Free carbenes are transient reactive intermediates in a broad range of organic transformations. A catalyst increases the rate of. Catalysis Organic Chemistry Reaction.
From www.slideserve.com
PPT CATALYSIS BY ACIDS AND BASES PowerPoint Presentation, free Catalysis Organic Chemistry Reaction This overview is arranged by catalyst class where the photophysics and electrochemical characteristics of each is discussed to underscore the differences and advantages to each type of single. Free carbenes are transient reactive intermediates in a broad range of organic transformations. First, we will examine the utility of photoredox catalysis from a historical viewpoint, and thereafter, we will discuss the. Catalysis Organic Chemistry Reaction.