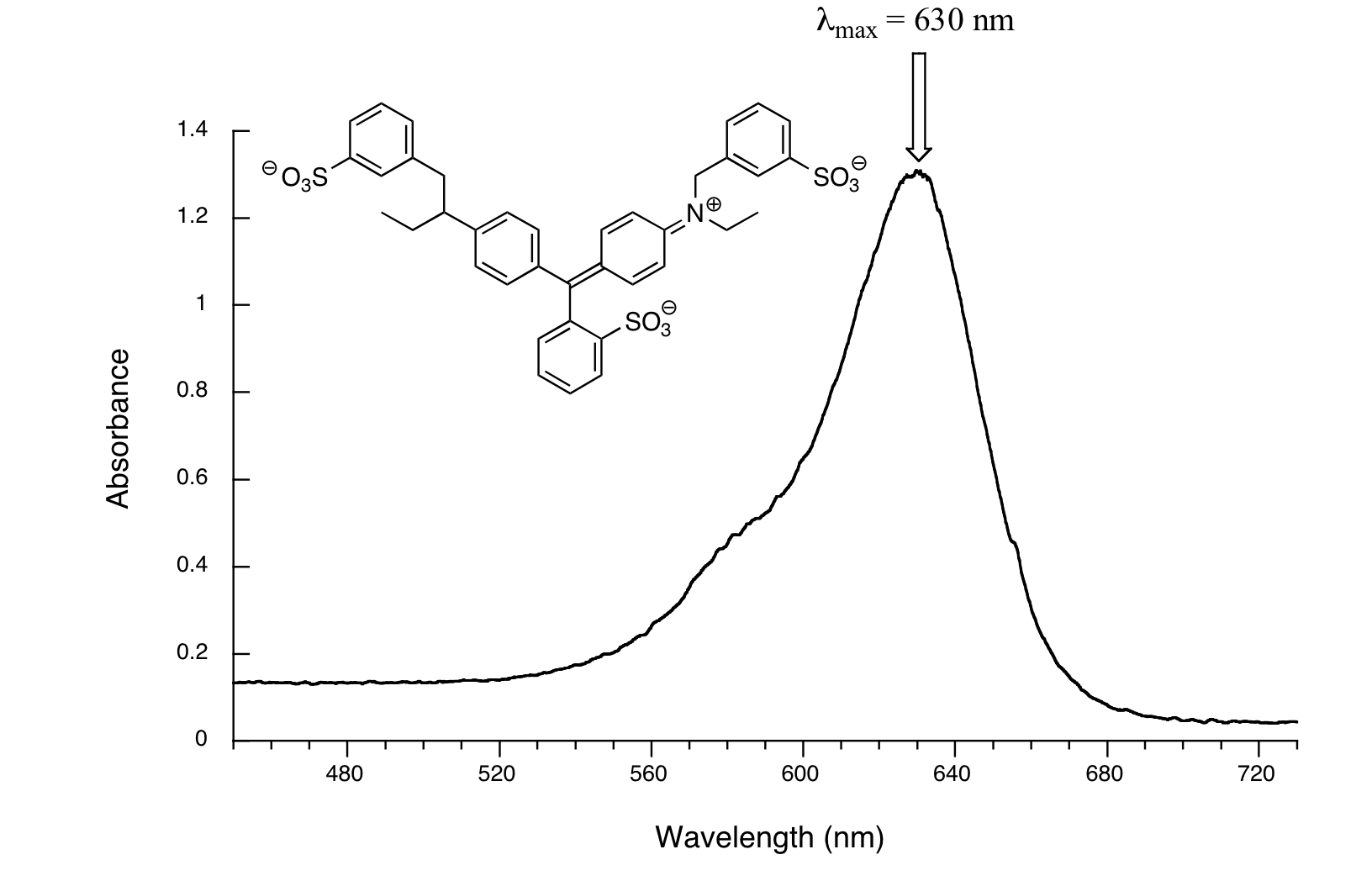
Uv Spectrophotometer Graph . Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique.
from www.analyzetest.com
In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals.
A to Z of UVVis spectroscopy interpretation
Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals.
From www.researchgate.net
UVVis Spectrophotometer Calibration Curve to Detect the Color Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Uv Spectrophotometer Graph.
From exyziiamz.blob.core.windows.net
How To Graph Spectrophotometer Reading at Amanda Pierson blog Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.jasco-global.com
Principles of UV/vis spectroscopy (7) Bandwidth JASCO Global Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From mavink.com
Uv Vis Absorption Chart Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From www.researchgate.net
Graph with absorption spectrum from UVVis spectrophotometer and the Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From americanlaboratorytrading.com
Thermo Scientific GENESYS 10 UVVisible Spectrophotometer Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Uv Spectrophotometer Graph.
From www.implen.de
Top UV Vis Spectrophotometer Data Management Implen NanoPhotometer Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.jove.com
UltravioletVisible (UVVis) Spectroscopy Definition JoVE Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.researchgate.net
UVvisible spectroscopy of phytosynthesized Copper nanoparticles using Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Uv Spectrophotometer Graph.
From www.analyzetest.com
A to Z of UVVis spectroscopy interpretation Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From www.researchgate.net
UVVis spectrophotometer test results. Download Scientific Diagram Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Uv Spectrophotometer Graph.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.researchgate.net
The spectrum of (a) ultravioletvisible (UVVis) spectrum and (b) curve Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From mungfali.com
UV Vis Spectroscopy Diagram Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.researchgate.net
UVvisible spectrophotometric analysis showed strong absorption peak Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Uv Spectrophotometer Graph.
From www.researchgate.net
UVVisible spectra for platinum nanoparticles, showing absorbance peak Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.researchgate.net
Colorimetric photograph of glucose assay, normalized UVvis spectra of Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.researchgate.net
A.2) Scan chart of UV. Spectrophotometer Download Scientific Diagram Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Uv Spectrophotometer Graph.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Ultraviolet spectroscopy Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From www.jasco-global.com
Principles of UV/vis spectroscopy (6) Baseline and blank JASCO Global Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.pinterest.co.uk
How a Spectrophotometer works! Infographic made by Hannah Hamel Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.researchgate.net
Ultravioletvisible diffuse reflectance spectroscopy (UVVis DRS Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From www.implen.de
Top UV Vis Spectrophotometer Data Management Implen NanoPhotometer Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Uv Spectrophotometer Graph.
From www.implen.de
Top UV Vis Spectrophotometer Data Management Implen NanoPhotometer Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From www.researchgate.net
Graph with absorption spectrum from UVVis spectrophotometer and the Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.researchgate.net
UVvisible spectrophotometer for the graphene film. Download Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.researchgate.net
Graph of data from UVVis Spectrophotometer. Download Scientific Diagram Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Uv Spectrophotometer Graph.
From chem.libretexts.org
4.4 UVVisible Spectroscopy Chemistry LibreTexts Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.
From www.researchgate.net
The Spectophotometry UV vis spectrum of ammonium hydroxide solution in Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From www.analyzetest.com
A to Z of UVVis spectroscopy interpretation Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From www.researchgate.net
UVVIS spectrophotometric graphs showing the optical behaviour of (a Uv Spectrophotometer Graph Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Uv Spectrophotometer Graph.
From www.researchgate.net
a Spectrum and b standard curve of UVVis spectrophotometer with Uv Spectrophotometer Graph What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From mavink.com
Uv Vis Spectral Range Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Software to analyze spectra, build density maps and molecular orbitals. Uv Spectrophotometer Graph.
From unimaterna.com.br
85/240VAC Inc. Digital Adjustment 325 to 1000nm Wavelength Range Laxco Uv Spectrophotometer Graph In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique. Software to analyze spectra, build density maps and molecular orbitals. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited state of the compound or material. Uv Spectrophotometer Graph.