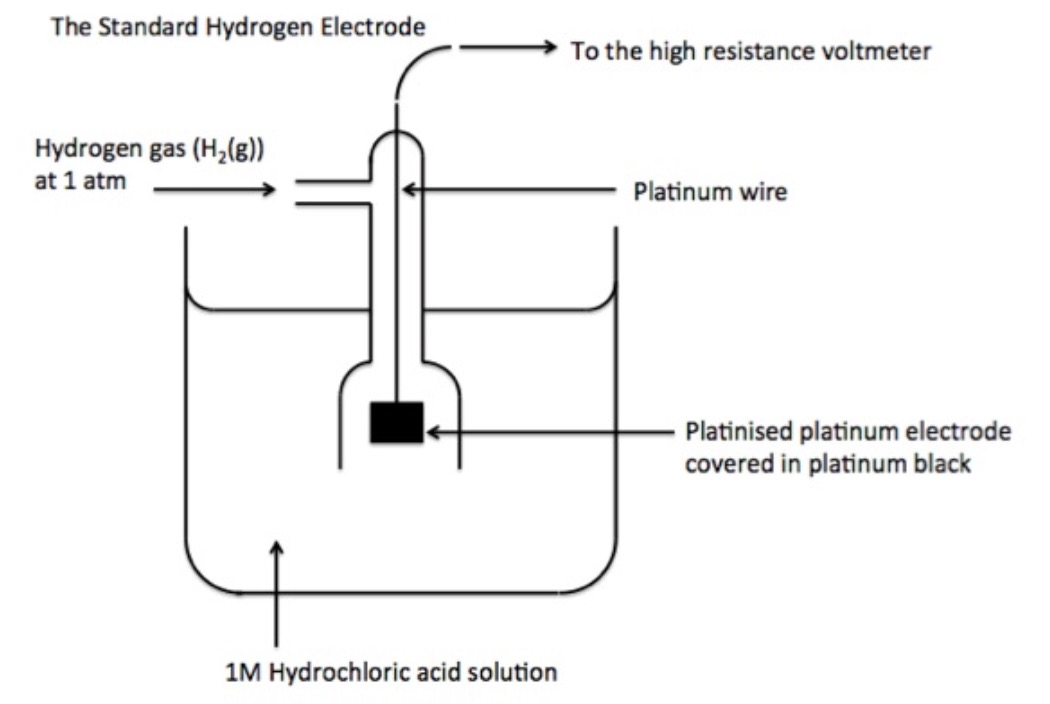
Electrodes A Level Chemistry . The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. An introduction to redox equilibria and electrode potentials. This flow of charged particles is. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. This page explains the background to standard electrode potentials. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. These are demonstrated using reversible half equations.
from classnotes.org.in
The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. This page explains the background to standard electrode potentials. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. This flow of charged particles is. An introduction to redox equilibria and electrode potentials. These are demonstrated using reversible half equations. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my.
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12, Electro Chemistry
Electrodes A Level Chemistry Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. These are demonstrated using reversible half equations. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. This flow of charged particles is. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Electrodes A Level Chemistry This page explains the background to standard electrode potentials. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Electrolysis is the decomposition of a molten or aqueous ionic. Electrodes A Level Chemistry.
From www.tes.com
AQA ALevel Chemistry Electrode Potentials and Electrochemical Cells A* Notes (New Spec Electrodes A Level Chemistry Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. An introduction to redox equilibria and electrode potentials. This flow of charged particles is. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Revision. Electrodes A Level Chemistry.
From www.tes.com
OCR Alevel Chemistry Redox and electrode potential Teaching Resources Electrodes A Level Chemistry This flow of charged particles is. This page explains the background to standard electrode potentials. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Revision notes on 5.3.3 standard electrode & cell potentials. Electrodes A Level Chemistry.
From www.savemyexams.co.uk
Electrode Potentials & Electrochemical Cells (A Level only) AQA A Level Chemistry Structured Electrodes A Level Chemistry These are demonstrated using reversible half equations. An introduction to redox equilibria and electrode potentials. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. This page explains the background to standard electrode potentials. The electrode (reduction) potential (e) is a value which shows how easily a substance. Electrodes A Level Chemistry.
From saylordotorg.github.io
Electrochemistry Electrodes A Level Chemistry Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my. Electrodes A Level Chemistry.
From www.tes.com
Predictions from electrode potentials A Level Chemistry for OCR A Chapter 23.5 Teaching Resources Electrodes A Level Chemistry Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. An introduction to redox equilibria and electrode potentials. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by. Electrodes A Level Chemistry.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts Electrodes A Level Chemistry This page explains the background to standard electrode potentials. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; These are demonstrated using reversible half equations. This flow of charged particles is.. Electrodes A Level Chemistry.
From mmerevise.co.uk
Electrochemical Cells MME Electrodes A Level Chemistry This flow of charged particles is. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; This page explains the background to standard electrode potentials. Electrolysis is the decomposition of a molten. Electrodes A Level Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrodes A Level Chemistry Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at. Electrodes A Level Chemistry.
From www.etsy.com
Electrode Etsy Electrodes A Level Chemistry These are demonstrated using reversible half equations. This flow of charged particles is. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. An introduction to redox equilibria and electrode potentials. Revision notes on. Electrodes A Level Chemistry.
From www.vrogue.co
A Level Aqa Chemistry Questions Electrode Potentials And Cells Revisely Vrogue Electrodes A Level Chemistry Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. This page explains the background to standard electrode potentials. Electrolysis is the decomposition. Electrodes A Level Chemistry.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry. YouTube Electrodes A Level Chemistry This flow of charged particles is. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; This page explains the background to standard electrode potentials. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Revision notes on 5.3.3 standard electrode & cell potentials. Electrodes A Level Chemistry.
From www.youtube.com
Electrochemistry 5 Standard hydrogen electrode YouTube Electrodes A Level Chemistry This page explains the background to standard electrode potentials. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. These are demonstrated using reversible half equations. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. The electrode (reduction). Electrodes A Level Chemistry.
From question.pandai.org
Standard Electrode Potential Electrodes A Level Chemistry This flow of charged particles is. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. These are demonstrated using reversible half equations. An introduction to redox equilibria and electrode potentials. This page explains the background to standard. Electrodes A Level Chemistry.
From www.youtube.com
CHEMISTRY A LEVEL ELECTRODE POTENTIAL YouTube Electrodes A Level Chemistry These are demonstrated using reversible half equations. This page explains the background to standard electrode potentials. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at. Electrodes A Level Chemistry.
From www.savemyexams.com
Standard Electrode & Cell Potentials CIE A Level Chemistry Revision Notes 2022 Electrodes A Level Chemistry Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. An introduction to redox equilibria and electrode potentials. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus,. Electrodes A Level Chemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrodes A Level Chemistry This flow of charged particles is. An introduction to redox equilibria and electrode potentials. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Electrochemical cells use redox reactions. Electrodes A Level Chemistry.
From www.hanlin.com
CIE A Level Chemistry复习笔记5.3.4 Measuring the Standard Electrode Potential翰林国际教育 Electrodes A Level Chemistry These are demonstrated using reversible half equations. An introduction to redox equilibria and electrode potentials. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Revision notes on. Electrodes A Level Chemistry.
From www.youtube.com
Electrode Potentials & Half Cells Alevel Chemistry OCR, AQA, Edexcel YouTube Electrodes A Level Chemistry This flow of charged particles is. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced;. Electrodes A Level Chemistry.
From www.vrogue.co
Aqa A Level Chemistry Electrode Potentials And Electr vrogue.co Electrodes A Level Chemistry This flow of charged particles is. This page explains the background to standard electrode potentials. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. An introduction to redox equilibria and electrode potentials. The. Electrodes A Level Chemistry.
From mungfali.com
Types Of Electrodes Electrodes A Level Chemistry This flow of charged particles is. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my.. Electrodes A Level Chemistry.
From classnotes.org.in
Electrochemical Series Chemistry, Class 12, Electro Chemistry Electrodes A Level Chemistry An introduction to redox equilibria and electrode potentials. This flow of charged particles is. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Electrolysis is the decomposition of. Electrodes A Level Chemistry.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Electrodes A Level Chemistry This page explains the background to standard electrode potentials. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; Electrolysis is the decomposition of a molten or aqueous ionic. Electrodes A Level Chemistry.
From www.youtube.com
Class 12 Electrochemistry / How electrode potential is measured by standard hydrogen electrode Electrodes A Level Chemistry An introduction to redox equilibria and electrode potentials. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. This page explains the background to standard electrode potentials. The electrode (reduction) potential (e) is a. Electrodes A Level Chemistry.
From www.awe-ltd.co.uk
Conductive level electrode range suitable for chemical usage Electrodes A Level Chemistry Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. An introduction to redox equilibria and electrode potentials. These are demonstrated using reversible half equations. This page explains the background to standard electrode potentials. This flow of charged particles is. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus,. Electrodes A Level Chemistry.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube Electrodes A Level Chemistry These are demonstrated using reversible half equations. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. This page explains the background to standard electrode potentials. Electrolysis is the decomposition of a. Electrodes A Level Chemistry.
From www.tes.com
OCR ALevel Chemistry Redox and Electrode Potentials Revision Notes Teaching Resources Electrodes A Level Chemistry This page explains the background to standard electrode potentials. An introduction to redox equilibria and electrode potentials. This flow of charged particles is. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Electrochemical cells use redox reactions as the electron transfer between products creates. Electrodes A Level Chemistry.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12, Electro Chemistry Electrodes A Level Chemistry The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; An introduction to redox equilibria and electrode potentials. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it.. Electrodes A Level Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrodes A Level Chemistry This flow of charged particles is. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. This page explains the background to standard electrode potentials. An introduction to redox equilibria and electrode. Electrodes A Level Chemistry.
From www.tes.com
Alevel AQA Chemistry electrode potentials Teaching Resources Electrodes A Level Chemistry Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. An introduction to redox equilibria and electrode potentials. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. This page explains the background to standard electrode potentials. The electrode (reduction) potential (e). Electrodes A Level Chemistry.
From www.vrogue.co
Aqa A Level Chemistry Electrode Potentials And Electr vrogue.co Electrodes A Level Chemistry This flow of charged particles is. This page explains the background to standard electrode potentials. Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. An introduction to redox equilibria and electrode potentials. Electrolysis is the decomposition of a molten or aqueous ionic compound (an. Electrodes A Level Chemistry.
From www.tes.com
OCR A Level Chemistry Redox and Electrode potential Cells Teaching Resources Electrodes A Level Chemistry The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; This flow of charged particles is. These are demonstrated using reversible half equations. An introduction to redox equilibria and electrode potentials. Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. Revision notes on. Electrodes A Level Chemistry.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Electrodes A Level Chemistry An introduction to redox equilibria and electrode potentials. Revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my. This flow of charged particles is. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; Revision notes on 5.3.3 standard electrode & cell. Electrodes A Level Chemistry.
From www.tes.com
Redox & Electrode Potentials (OCR A Level Chemistry) Teaching Resources Electrodes A Level Chemistry Revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; This flow of. Electrodes A Level Chemistry.
From www.studypool.com
SOLUTION Types of electrodes chemistry Studypool Electrodes A Level Chemistry The electrode (reduction) potential (e) is a value which shows how easily a substance is reduced; Electrolysis is the decomposition of a molten or aqueous ionic compound (an electrolyte) by passing an electric current through it. These are demonstrated using reversible half equations. This flow of charged particles is. This page explains the background to standard electrode potentials. Revision notes. Electrodes A Level Chemistry.