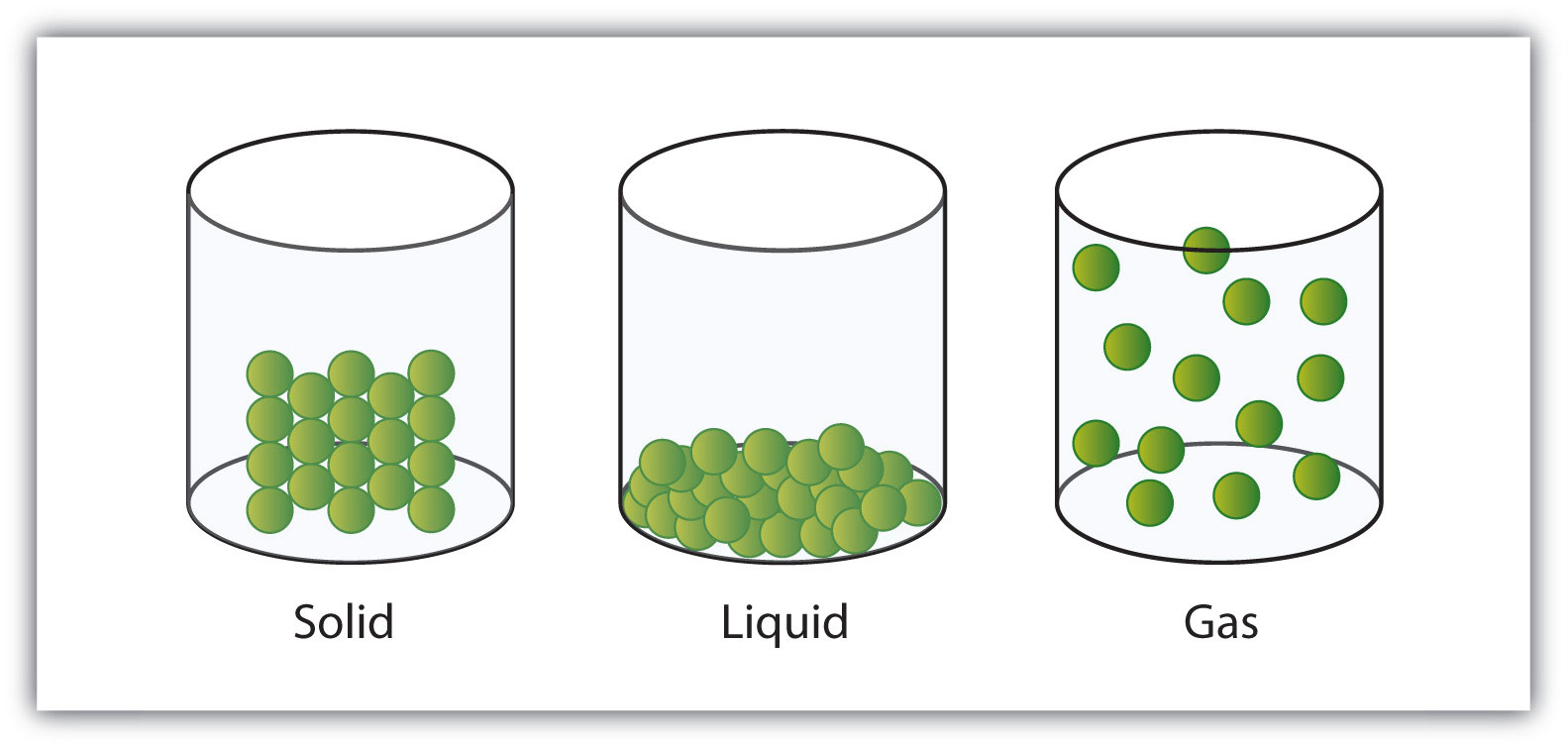
Solids Liquids And Solutions . Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. Students are introduced to the distinctive properties of mixtures and solutions. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. A class demonstration led by the. The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions.
from courses.lumenlearning.com
On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. A class demonstration led by the. Students are introduced to the distinctive properties of mixtures and solutions. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together.
8.2 Solids and Liquids The Basics of General, Organic, and Biological Chemistry
Solids Liquids And Solutions Students are introduced to the distinctive properties of mixtures and solutions. Students are introduced to the distinctive properties of mixtures and solutions. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. A class demonstration led by the.
From libguides.bbc.qld.edu.au
States of Matter Junior School Brisbane Boys' College Library at Brisbane Boys' College Solids Liquids And Solutions A class demonstration led by the. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. The physical properties of condensed matter (liquids and solids) can be explained in terms. Solids Liquids And Solutions.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solids Liquids And Solutions Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. A class demonstration led by the. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases. Solids Liquids And Solutions.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solids Liquids And Solutions On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Students are introduced to the distinctive properties of mixtures and solutions. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. The physical properties of condensed matter (liquids and solids) can be. Solids Liquids And Solutions.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solids Liquids And Solutions Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Students are introduced to the distinctive. Solids Liquids And Solutions.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Illustration Solids Liquids And Solutions A class demonstration led by the. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases. Solids Liquids And Solutions.
From www.slideserve.com
PPT Solids , Liquids and Solutions PowerPoint Presentation, free download ID9401280 Solids Liquids And Solutions Students are introduced to the distinctive properties of mixtures and solutions. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Although the word solution is commonly applied to the liquid state. Solids Liquids And Solutions.
From sciencenotes.org
10 Examples of Solids, Liquids, Gases, and Plasma Solids Liquids And Solutions Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. A class demonstration. Solids Liquids And Solutions.
From www.pinterest.com
Task Cards States of Matter Solids, Liquids, Gases Their Properties & Solutions For Grades Solids Liquids And Solutions A class demonstration led by the. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are. Solids Liquids And Solutions.
From www.studypool.com
SOLUTION Examples of solid liquid gas Studypool Solids Liquids And Solutions On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. A class demonstration led by the. Students are introduced to the distinctive properties of mixtures and solutions. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. Although the word solution is commonly applied. Solids Liquids And Solutions.
From socratic.org
What are the characteristics of a solid? + Example Solids Liquids And Solutions Students are introduced to the distinctive properties of mixtures and solutions. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. Although the word solution is commonly applied to the liquid state of matter, solutions. Solids Liquids And Solutions.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Scheme Stock Vector Solids Liquids And Solutions Students are introduced to the distinctive properties of mixtures and solutions. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. A class demonstration led by the. On the basis of physical states of. Solids Liquids And Solutions.
From www.pinterest.co.uk
Solutions Matter science, Matter worksheets, States of matter worksheet Solids Liquids And Solutions On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. In contrast to gases, solids and. Solids Liquids And Solutions.
From www.askiitians.com
General Characteristics of Solid State Study Material for IIT JEE askIITians Solids Liquids And Solutions The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. A class demonstration led by the. Students are introduced to the distinctive properties of mixtures and solutions. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. In contrast to gases, solids and. Solids Liquids And Solutions.
From www.pinterest.com
Coverage of this EDITABLE All in 1 Pack A. What is Matter? B. States of matter Solid, Liquid Solids Liquids And Solutions The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. A class demonstration led by the.. Solids Liquids And Solutions.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solids Liquids And Solutions Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. On the basis. Solids Liquids And Solutions.
From www.youtube.com
Science Lesson 4 Mixing Solids into Liquids YouTube Solids Liquids And Solutions The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Although the word solution. Solids Liquids And Solutions.
From www.slideserve.com
PPT Solids , Liquids and Solutions PowerPoint Presentation, free download ID4501528 Solids Liquids And Solutions In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. Students are introduced to the distinctive properties of mixtures and solutions. Solutions are homogeneous mixtures of two or more substances. Solids Liquids And Solutions.
From www.dreamstime.com
Solution Solid in liquid stock vector. Illustration of mixtures 102286653 Solids Liquids And Solutions In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Students are introduced to the distinctive properties of mixtures and solutions. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. The physical properties of condensed matter (liquids and solids) can be explained in. Solids Liquids And Solutions.
From www.dreamstime.com
Solution. Solid in liquid stock vector. Illustration of expansion 67386593 Solids Liquids And Solutions Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. A class demonstration led by the. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are. Solids Liquids And Solutions.
From mavink.com
Parts Of Matter Solids Liquids And Solutions In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. The. Solids Liquids And Solutions.
From examples.yourdictionary.com
Common Examples of Solutions Science in Everyday Life YourDictionary Solids Liquids And Solutions In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. A class demonstration led by the. Students are introduced to the distinctive properties of mixtures and solutions. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. Solutions are homogeneous. Solids Liquids And Solutions.
From www.tutorix.com
Give three characteristics of solid liquid and gas Tutorix Solids Liquids And Solutions In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. Students are introduced to the distinctive properties of mixtures and solutions. The physical properties of condensed matter (liquids and solids) can be explained in. Solids Liquids And Solutions.
From webmis.highland.cc.il.us
Aqueous Solutions Solids Liquids And Solutions Students are introduced to the distinctive properties of mixtures and solutions. The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. On the basis of physical states of solvent and solute. Solids Liquids And Solutions.
From www.slideserve.com
PPT Unit 11 Liquids, Solids, and Solutions PowerPoint Presentation, free download ID3593121 Solids Liquids And Solutions Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Students are introduced to the distinctive properties of mixtures and solutions. Although the word solution is commonly applied to the liquid state of matter,. Solids Liquids And Solutions.
From studylib.net
States of Matter Solids Liquids And Solutions In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. A class demonstration led by the. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. The physical properties of condensed matter (liquids and solids) can be explained in terms of the. Solids Liquids And Solutions.
From www.youtube.com
Solids, Liquids and Gases Class 5 SCIENCE CBSE/NCERT Solutions Three States of Matter Solids Liquids And Solutions The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. Students are introduced to the distinctive. Solids Liquids And Solutions.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? Solids Liquids And Solutions Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. A class demonstration led by the. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous. Solids Liquids And Solutions.
From www.youtube.com
Solid in liquid solution YouTube Solids Liquids And Solutions Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. On the basis. Solids Liquids And Solutions.
From www.etsy.com
Science, States of Matter, Solid, Liquid, Gas, Elementary, Anchor Chart, Educational Poster Etsy Solids Liquids And Solutions On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Although the word solution is. Solids Liquids And Solutions.
From dokumen.tips
(PDF) Unit 6 Solids, Liquids and Solutions CourseNotes · Unit 6 Solids, Liquids and Solids Liquids And Solutions Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. A class demonstration led by the. In contrast to gases, solids and liquids have microscopic structures in which the constituent. Solids Liquids And Solutions.
From primaryleap.co.uk
Chemistry Solutions And Mixtures Level 1 activity for kids PrimaryLeap.co.uk Solids Liquids And Solutions The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. A class demonstration led. Solids Liquids And Solutions.
From www.snexplores.org
Explainer What are the different states of matter? Solids Liquids And Solutions In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Solutions. Solids Liquids And Solutions.
From diagramlibraryconjoin.z19.web.core.windows.net
Solid Liquid And Gas Diagram Solids Liquids And Solutions In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. A class demonstration led by the. Students are introduced to the distinctive properties of mixtures and solutions. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. The physical properties of condensed. Solids Liquids And Solutions.
From courses.lumenlearning.com
8.2 Solids and Liquids The Basics of General, Organic, and Biological Chemistry Solids Liquids And Solutions The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. In contrast to gases, solids and liquids have microscopic structures in which the constituent particles are very close together. A class demonstration led by the. Solutions are homogeneous mixtures of two or more substances whose components are uniformly distributed on a microscopic.. Solids Liquids And Solutions.
From www.slideserve.com
PPT Unit 5 Solids, Liquids, and Solutions PowerPoint Presentation, free download ID1011545 Solids Liquids And Solutions On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. The physical properties of condensed matter (liquids and solids) can be explained in terms of the kinetic molecular. Although the word solution is commonly applied to the liquid state of matter, solutions of solids and gases are also possible;. Students are. Solids Liquids And Solutions.