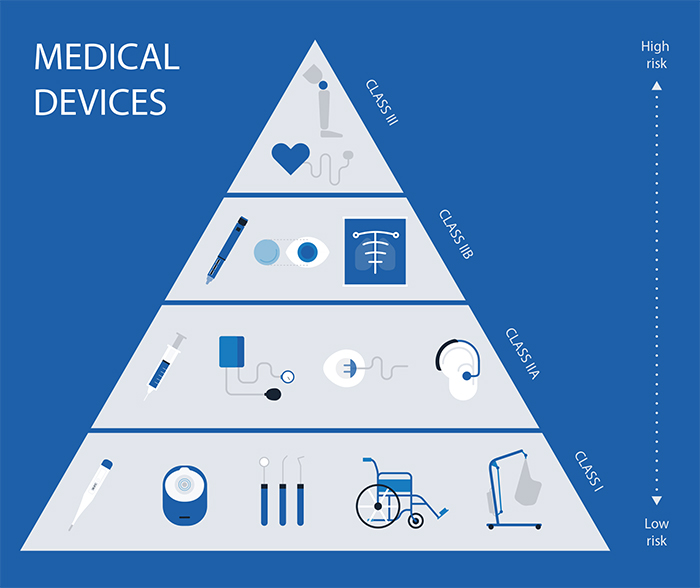
Classification Of Medical Devices With Examples . A medical device within the u.s. The fda classifies medical devices. The united states food and drug administration (fda) categorizes medical devices into three classes: Blood glucose meters, hearing aids, and nebulisers. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Class i, class ii, or class iii.
from laegemiddelstyrelsen.dk
Class i, class ii, or class iii. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Blood glucose meters, hearing aids, and nebulisers. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The united states food and drug administration (fda) categorizes medical devices into three classes: The fda classifies medical devices. A medical device within the u.s.
Medical devices
Classification Of Medical Devices With Examples The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. The fda classifies medical devices. Blood glucose meters, hearing aids, and nebulisers. A medical device within the u.s. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. The united states food and drug administration (fda) categorizes medical devices into three classes: Class i, class ii, or class iii. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Classification Of Medical Devices With Examples The fda classifies medical devices. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. A medical device within the u.s. Blood glucose meters, hearing aids, and nebulisers. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices. Classification Of Medical Devices With Examples.
From lifechanginginnovation.org
What is a Medical Device? Life Changing Innovation Classification Of Medical Devices With Examples The united states food and drug administration (fda) categorizes medical devices into three classes: The fda classifies medical devices. Class i, class ii, or class iii. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Blood glucose meters, hearing aids, and nebulisers. A medical device within the. Classification Of Medical Devices With Examples.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Classification Of Medical Devices With Examples Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. Classification of a medical device (21 cfr 860) medical devices are regulated based. Classification Of Medical Devices With Examples.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Classification Of Medical Devices With Examples Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. The united states food and drug administration (fda) categorizes medical devices into three classes: Section 201(h) of the fdca defines. Classification Of Medical Devices With Examples.
From www.pacificbridgemedical.com
Classification of General Medical Devices in Singapore Classification Of Medical Devices With Examples The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. A medical device within the u.s. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Blood glucose meters, hearing aids, and nebulisers. The united states food and drug administration. Classification Of Medical Devices With Examples.
From omcmedical.com
Classification of Medical Devices Based on UK MDR 2002 Classification Of Medical Devices With Examples The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. The united states food and drug administration (fda) categorizes medical devices into three classes: Class i, class ii, or class iii. The food and drug administration (fda) has established classifications for approximately 1,700 different generic. Classification Of Medical Devices With Examples.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Classification Of Medical Devices With Examples The fda classifies medical devices. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Blood glucose meters, hearing aids, and nebulisers. Class i, class ii, or class iii. The. Classification Of Medical Devices With Examples.
From www.pacificbridgemedical.com
Device Classification in India Infographic Classification Of Medical Devices With Examples Class i, class ii, or class iii. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. A medical device within the u.s. Classification of a medical. Classification Of Medical Devices With Examples.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Classification Of Medical Devices With Examples A medical device within the u.s. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Class i, class ii, or class iii. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Blood glucose meters, hearing aids, and nebulisers.. Classification Of Medical Devices With Examples.
From www.medicalmicromolding.com
UK Medical Device Classification Classification Of Medical Devices With Examples Class i, class ii, or class iii. The fda classifies medical devices. The united states food and drug administration (fda) categorizes medical devices into three classes: A medical device within the u.s. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Blood glucose meters, hearing aids, and. Classification Of Medical Devices With Examples.
From es.slideshare.net
Regulation of Medical Devices in US Classification Of Medical Devices With Examples Blood glucose meters, hearing aids, and nebulisers. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. Classification of a medical device (21 cfr 860) medical devices are regulated based. Classification Of Medical Devices With Examples.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Classification Of Medical Devices With Examples Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. The united states food and drug administration (fda) categorizes medical devices into three. Classification Of Medical Devices With Examples.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Classification Of Medical Devices With Examples The united states food and drug administration (fda) categorizes medical devices into three classes: A medical device within the u.s. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Blood glucose meters, hearing aids, and nebulisers. The food and drug administration (fda) has established classifications for approximately. Classification Of Medical Devices With Examples.
From angelanjohnson.com
Medical Devices Angela N Johnson Classification Of Medical Devices With Examples The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Blood glucose meters, hearing aids, and nebulisers. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The united states food and drug administration (fda) categorizes medical devices into three. Classification Of Medical Devices With Examples.
From www.vrogue.co
Classification Of Medical Devices Pharmacovigilance vrogue.co Classification Of Medical Devices With Examples The united states food and drug administration (fda) categorizes medical devices into three classes: The fda classifies medical devices. Class i, class ii, or class iii. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. Section 201(h) of the fdca defines a medical device. Classification Of Medical Devices With Examples.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Classification Of Medical Devices With Examples The united states food and drug administration (fda) categorizes medical devices into three classes: A medical device within the u.s. Class i, class ii, or class iii. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. Classification of a medical device (21 cfr 860). Classification Of Medical Devices With Examples.
From chinameddevice.com
CFDA New Medical Device Classification Catalogue Effective August 1st Classification Of Medical Devices With Examples A medical device within the u.s. Blood glucose meters, hearing aids, and nebulisers. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. The fda classifies medical. Classification Of Medical Devices With Examples.
From www.researchgate.net
Medical Device Classification System Download Table Classification Of Medical Devices With Examples A medical device within the u.s. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. The united states food and drug administration. Classification Of Medical Devices With Examples.
From www.presentationeze.com
FDA medical device classification PresentationEZE Classification Of Medical Devices With Examples The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. The fda classifies medical devices. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. Blood glucose meters, hearing aids, and nebulisers. Section 201(h) of the fdca. Classification Of Medical Devices With Examples.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Classification Of Medical Devices With Examples Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. The fda classifies medical devices. Section 201(h) of the fdca defines a medical device as any product that does not. Classification Of Medical Devices With Examples.
From laegemiddelstyrelsen.dk
Classification of in vitro diagnostic medical devices (IVD) Classification Of Medical Devices With Examples Blood glucose meters, hearing aids, and nebulisers. The united states food and drug administration (fda) categorizes medical devices into three classes: Class i, class ii, or class iii. A medical device within the u.s. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. The. Classification Of Medical Devices With Examples.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Classification Of Medical Devices With Examples The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. A medical device within the u.s. Blood glucose meters, hearing aids, and nebulisers. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or.. Classification Of Medical Devices With Examples.
From operonstrategist.com
Examples of Medical Devices as per Classifications Operon Strategist Classification Of Medical Devices With Examples The united states food and drug administration (fda) categorizes medical devices into three classes: The fda classifies medical devices. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. A medical device within the u.s. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk. Classification Of Medical Devices With Examples.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Classification Of Medical Devices With Examples Blood glucose meters, hearing aids, and nebulisers. Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. A medical device within the u.s. The purpose of. Classification Of Medical Devices With Examples.
From medicaldevicehq.com
Different classifications rules for medical device software An Classification Of Medical Devices With Examples Class i, class ii, or class iii. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. A medical device within the u.s. Blood glucose meters, hearing. Classification Of Medical Devices With Examples.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Classification Of Medical Devices With Examples Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. A medical device within the u.s. The food and drug administration (fda) has established classifications for. Classification Of Medical Devices With Examples.
From laegemiddelstyrelsen.dk
Medical devices Classification Of Medical Devices With Examples Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. A medical device within the u.s. The fda classifies medical devices. The purpose of this chapter is to provide a. Classification Of Medical Devices With Examples.
From www.researchgate.net
3 Examples of medical devices according to the European and USA Classification Of Medical Devices With Examples Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. Class i, class ii, or class iii. A medical device within the u.s. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The fda classifies. Classification Of Medical Devices With Examples.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Classification Of Medical Devices With Examples Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. The united states food and drug administration (fda) categorizes medical devices into three classes: Class i, class ii, or class. Classification Of Medical Devices With Examples.
From mavink.com
Mdr Classification Chart Classification Of Medical Devices With Examples The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. A medical device within the u.s. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Blood glucose meters, hearing aids, and nebulisers. Section 201(h) of the. Classification Of Medical Devices With Examples.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Classification Of Medical Devices With Examples Class i, class ii, or class iii. Blood glucose meters, hearing aids, and nebulisers. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. The fda classifies medical devices. A medical device within the u.s. The united states food and drug administration (fda) categorizes medical devices into three classes: Classification of a. Classification Of Medical Devices With Examples.
From mungfali.com
Classification Of Medical Devices Classification Of Medical Devices With Examples Section 201(h) of the fdca defines a medical device as any product that does not achieve its purposes by chemical action or. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the. Classification Of Medical Devices With Examples.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Classification Of Medical Devices With Examples Blood glucose meters, hearing aids, and nebulisers. The united states food and drug administration (fda) categorizes medical devices into three classes: The fda classifies medical devices. Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Class i, class ii, or class iii. A medical device within the. Classification Of Medical Devices With Examples.
From smartdataweek.com
Medical Device Classification (FDA & EU MDR) SimplerQMS (2024) Classification Of Medical Devices With Examples Classification of a medical device (21 cfr 860) medical devices are regulated based on the relative risk posed by the product and. Class i, class ii, or class iii. The united states food and drug administration (fda) categorizes medical devices into three classes: The fda classifies medical devices. The purpose of this chapter is to provide a general overview on. Classification Of Medical Devices With Examples.
From www.arenasolutions.com
How to Classify Your Medical Device Under the EU MDR and IVDR Arena Classification Of Medical Devices With Examples The fda classifies medical devices. The purpose of this chapter is to provide a general overview on the impact of the classification of medical devices on different aspects of the. Blood glucose meters, hearing aids, and nebulisers. The food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices and. Section 201(h) of the fdca. Classification Of Medical Devices With Examples.