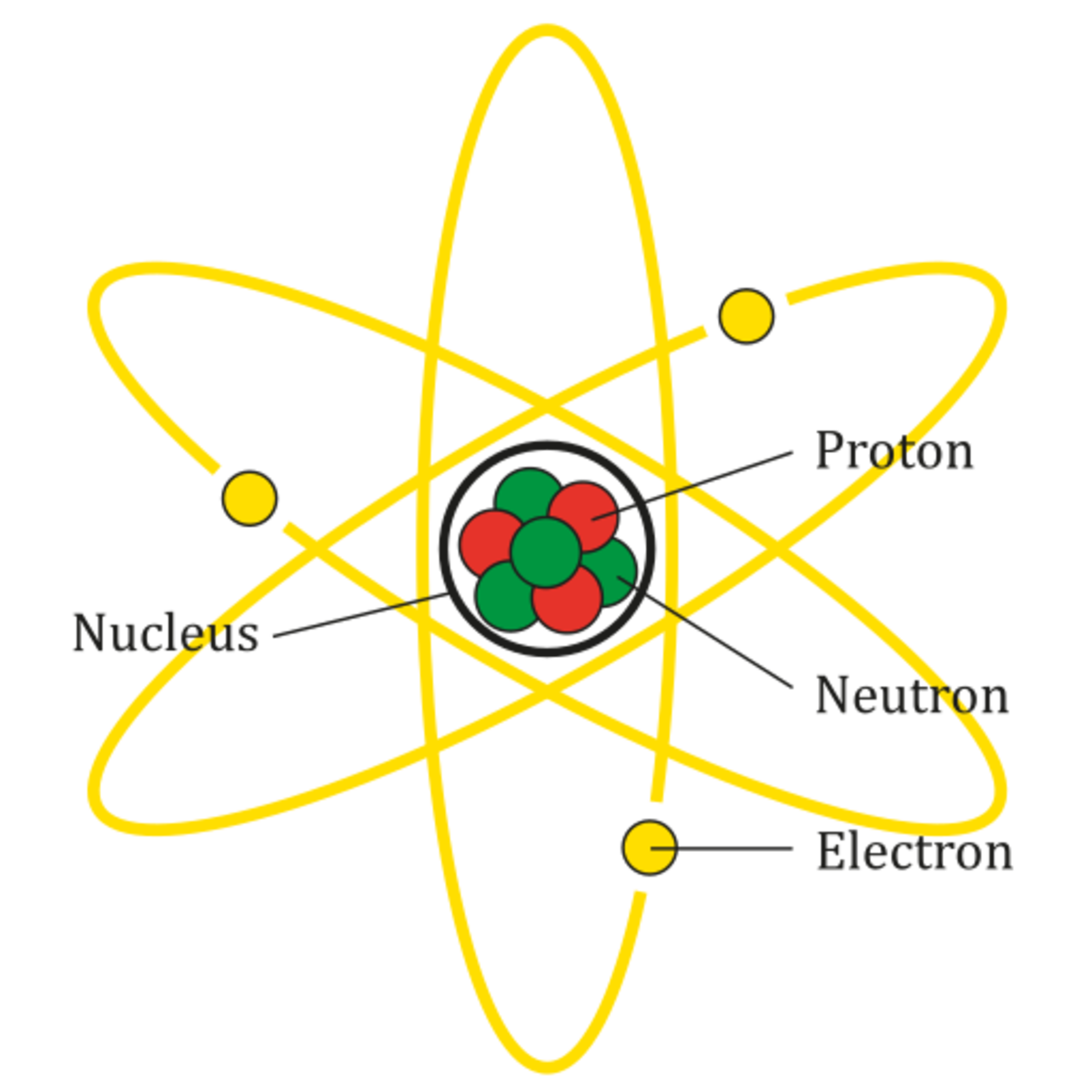
How Small Is A Nucleus Compared To An Atom . The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. The nucleus of the atom is extremely small. To put it another way, an atom the size of a football field has a nucleus the side of a pea. The nucleus of an atom forms its tiny core, with a radius 10,000 to. Its radius is only about 1/100,000 of the total radius of the atom. Of an atom is less than. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. They are formed of a nucleus, protons, and electrons. The nucleus is tiny when compared to the size of an atom. The nucleus is tiny compared to the atom as a whole: The atomic nucleus is extremely tiny, yet very dense. Atoms make up everything and are incredibly small. It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the atom.
from owlcation.com
They are formed of a nucleus, protons, and electrons. Atoms make up everything and are incredibly small. The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. To put it another way, an atom the size of a football field has a nucleus the side of a pea. The atomic nucleus is extremely tiny, yet very dense. Of an atom is less than. It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the atom. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. Its radius is only about 1/100,000 of the total radius of the atom. The nucleus of the atom is extremely small.
Atoms, Molecules, and Compounds What's the Difference? Owlcation
How Small Is A Nucleus Compared To An Atom They are formed of a nucleus, protons, and electrons. It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the atom. The nucleus is tiny compared to the atom as a whole: Of an atom is less than. The nucleus of the atom is extremely small. Its radius is only about 1/100,000 of the total radius of the atom. The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. To put it another way, an atom the size of a football field has a nucleus the side of a pea. The atomic nucleus is extremely tiny, yet very dense. The nucleus of an atom forms its tiny core, with a radius 10,000 to. The nucleus is tiny when compared to the size of an atom. They are formed of a nucleus, protons, and electrons. Atoms make up everything and are incredibly small.
From eduinput.com
Atomic Nucleus Nucleons, Charge Number, and Mass Number How Small Is A Nucleus Compared To An Atom The nucleus of an atom forms its tiny core, with a radius 10,000 to. The nucleus is tiny compared to the atom as a whole: Of an atom is less than. The nucleus of the atom is extremely small. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. The. How Small Is A Nucleus Compared To An Atom.
From www.youtube.com
The Size of the Atomic Nucleus YouTube How Small Is A Nucleus Compared To An Atom The nucleus of the atom is extremely small. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. The atomic nucleus is extremely tiny, yet very dense. Its radius is only about 1/100,000 of the total radius of the atom. They are formed of a nucleus, protons, and electrons. Atoms. How Small Is A Nucleus Compared To An Atom.
From slideplayer.com
Physical Science Chemistry Unit 2 ppt download How Small Is A Nucleus Compared To An Atom Its radius is only about 1/100,000 of the total radius of the atom. To put it another way, an atom the size of a football field has a nucleus the side of a pea. Of an atom is less than. The nucleus is tiny when compared to the size of an atom. The nucleus of the atom is extremely small.. How Small Is A Nucleus Compared To An Atom.
From pixabay.com
Atom Nucleus Nuclear · Free vector graphic on Pixabay How Small Is A Nucleus Compared To An Atom Its radius is only about 1/100,000 of the total radius of the atom. The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. The nucleus of an atom forms its tiny core, with a radius 10,000 to. Of an atom is less than. The nucleus is. How Small Is A Nucleus Compared To An Atom.
From www.chem.fsu.edu
Electron Configurations How Small Is A Nucleus Compared To An Atom Of an atom is less than. It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the atom. The nucleus is tiny compared to the atom as a whole: Atoms make up everything and are incredibly small. The nucleus of the atom is extremely small. The atomic nucleus is extremely tiny,. How Small Is A Nucleus Compared To An Atom.
From www.vedantu.com
State the differences between Nucleus and Nucleolus. How Small Is A Nucleus Compared To An Atom It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the atom. The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. Atoms make up everything and are incredibly small. The nucleus is tiny when compared to. How Small Is A Nucleus Compared To An Atom.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definition [with Examples] How Small Is A Nucleus Compared To An Atom The nucleus of the atom is extremely small. Its radius is only about 1/100,000 of the total radius of the atom. Atoms make up everything and are incredibly small. The nucleus is tiny compared to the atom as a whole: Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,.. How Small Is A Nucleus Compared To An Atom.
From sciencenotes.org
Periodic Table of Element Atom Sizes How Small Is A Nucleus Compared To An Atom Atoms make up everything and are incredibly small. The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. Its radius is only about 1/100,000 of the total radius of the atom. The nucleus of the atom is extremely small. The nucleus of an atom forms its. How Small Is A Nucleus Compared To An Atom.
From sciencenotes.org
Atomic Nucleus Definition and Facts How Small Is A Nucleus Compared To An Atom The nucleus of the atom is extremely small. The atomic nucleus is extremely tiny, yet very dense. Its radius is only about 1/100,000 of the total radius of the atom. It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the atom. The nucleus is tiny compared to the atom as. How Small Is A Nucleus Compared To An Atom.
From www.slideserve.com
PPT CHEMISTRY The Molecular Science Chapter two PowerPoint How Small Is A Nucleus Compared To An Atom The atomic nucleus is extremely tiny, yet very dense. The nucleus of an atom forms its tiny core, with a radius 10,000 to. The nucleus is tiny when compared to the size of an atom. They are formed of a nucleus, protons, and electrons. It is true the nucleus is a loose cluster of protons and neutrons, but it is. How Small Is A Nucleus Compared To An Atom.
From www.pathwaystochemistry.com
Atomic Structure Pathways to Chemistry How Small Is A Nucleus Compared To An Atom The atomic nucleus is extremely tiny, yet very dense. The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. The nucleus of an atom forms its tiny core, with a radius 10,000 to. The nucleus is tiny compared to the atom as a whole: To put. How Small Is A Nucleus Compared To An Atom.
From slideplayer.com
Modern Theories of the Atom ppt download How Small Is A Nucleus Compared To An Atom They are formed of a nucleus, protons, and electrons. The nucleus is tiny compared to the atom as a whole: To put it another way, an atom the size of a football field has a nucleus the side of a pea. It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to. How Small Is A Nucleus Compared To An Atom.
From www.shalom-education.com
Atomic Structure and Ions GCSE Chemistry Revision How Small Is A Nucleus Compared To An Atom Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. They are formed of a nucleus, protons, and electrons. The nucleus is tiny when compared to. How Small Is A Nucleus Compared To An Atom.
From slideplayer.com
Modern Theories of the Atom ppt download How Small Is A Nucleus Compared To An Atom The nucleus is tiny compared to the atom as a whole: The atomic nucleus is extremely tiny, yet very dense. The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. The nucleus is tiny when compared to the size of an atom. To put it another. How Small Is A Nucleus Compared To An Atom.
From www.sciencefacts.net
Atomic Nucleus Definition, Structure & Parts with Diagram How Small Is A Nucleus Compared To An Atom Atoms make up everything and are incredibly small. The atomic nucleus is extremely tiny, yet very dense. The nucleus is tiny compared to the atom as a whole: To put it another way, an atom the size of a football field has a nucleus the side of a pea. They are formed of a nucleus, protons, and electrons. The atomic. How Small Is A Nucleus Compared To An Atom.
From www.expii.com
Atoms — Definition & Overview Expii How Small Is A Nucleus Compared To An Atom The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. The nucleus of the atom is extremely small. The nucleus is tiny when compared to the size of an atom. The nucleus is tiny compared to the atom as a whole: It is true the nucleus. How Small Is A Nucleus Compared To An Atom.
From www.youtube.com
The Nucleolus (the small nucleus) YouTube How Small Is A Nucleus Compared To An Atom The nucleus is tiny compared to the atom as a whole: The nucleus is tiny when compared to the size of an atom. It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the atom. The atomic nucleus is extremely tiny, yet very dense. They are formed of a nucleus, protons,. How Small Is A Nucleus Compared To An Atom.
From slideplayer.com
Atomic Structure Notes ppt download How Small Is A Nucleus Compared To An Atom They are formed of a nucleus, protons, and electrons. The atomic nucleus is extremely tiny, yet very dense. To put it another way, an atom the size of a football field has a nucleus the side of a pea. The nucleus of the atom is extremely small. Of an atom is less than. It is true the nucleus is a. How Small Is A Nucleus Compared To An Atom.
From ablogwithadifference.com
Between Cell and Atom best 5 difference How Small Is A Nucleus Compared To An Atom To put it another way, an atom the size of a football field has a nucleus the side of a pea. Of an atom is less than. It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the atom. Its radius is only about 1/100,000 of the total radius of the. How Small Is A Nucleus Compared To An Atom.
From www.broadlearnings.com
Atomic Structure Broad Learnings How Small Is A Nucleus Compared To An Atom Atoms make up everything and are incredibly small. The nucleus is tiny when compared to the size of an atom. To put it another way, an atom the size of a football field has a nucleus the side of a pea. The atomic nucleus is extremely tiny, yet very dense. The nucleus of an atom forms its tiny core, with. How Small Is A Nucleus Compared To An Atom.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definitions.. and more How Small Is A Nucleus Compared To An Atom Its radius is only about 1/100,000 of the total radius of the atom. The nucleus is tiny when compared to the size of an atom. They are formed of a nucleus, protons, and electrons. The nucleus is tiny compared to the atom as a whole: It is true the nucleus is a loose cluster of protons and neutrons, but it. How Small Is A Nucleus Compared To An Atom.
From spmphysics.onlinetuition.com.my
The Composition of the Nucleus SPM Physics Form 4/Form 5 Revision Notes How Small Is A Nucleus Compared To An Atom The nucleus is tiny when compared to the size of an atom. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. The nucleus of an atom forms its tiny core, with a radius 10,000 to. It is true the nucleus is a loose cluster of protons and neutrons, but. How Small Is A Nucleus Compared To An Atom.
From www.nuclear-power.com
Atomic and Nuclear Structure Definition & Characteristics nuclear How Small Is A Nucleus Compared To An Atom The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. They are formed of a nucleus, protons, and electrons. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. Atoms make up everything and are incredibly. How Small Is A Nucleus Compared To An Atom.
From www.cyberphysics.co.uk
The nucleus How Small Is A Nucleus Compared To An Atom To put it another way, an atom the size of a football field has a nucleus the side of a pea. The nucleus is tiny when compared to the size of an atom. The atomic nucleus is extremely tiny, yet very dense. Its radius is only about 1/100,000 of the total radius of the atom. The nucleus is tiny compared. How Small Is A Nucleus Compared To An Atom.
From in.pinterest.com
A Demonstration of How Small Atomic Nuclei Are AtomicNucleus How Small Is A Nucleus Compared To An Atom The nucleus of an atom forms its tiny core, with a radius 10,000 to. It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the atom. The nucleus of the atom is extremely small. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the. How Small Is A Nucleus Compared To An Atom.
From slideshare.net
Basic Atomic Structure How Small Is A Nucleus Compared To An Atom They are formed of a nucleus, protons, and electrons. The nucleus of an atom forms its tiny core, with a radius 10,000 to. The atomic nucleus is extremely tiny, yet very dense. Of an atom is less than. The nucleus of the atom is extremely small. The atomic nucleus is the small, dense region consisting of protons and neutrons at. How Small Is A Nucleus Compared To An Atom.
From www.thoughtco.com
Basic Model of the Atom Atomic Theory How Small Is A Nucleus Compared To An Atom The nucleus is tiny when compared to the size of an atom. The nucleus of an atom forms its tiny core, with a radius 10,000 to. The nucleus of the atom is extremely small. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. Atoms make up everything and are. How Small Is A Nucleus Compared To An Atom.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer How Small Is A Nucleus Compared To An Atom The nucleus is tiny when compared to the size of an atom. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. The nucleus is tiny compared to the atom as a whole: The atomic nucleus is extremely tiny, yet very dense. Its radius is only about 1/100,000 of the. How Small Is A Nucleus Compared To An Atom.
From slideplayer.com
Introductory Chemistry, 3rd Edition ppt download How Small Is A Nucleus Compared To An Atom Its radius is only about 1/100,000 of the total radius of the atom. To put it another way, an atom the size of a football field has a nucleus the side of a pea. Atoms make up everything and are incredibly small. The nucleus of the atom is extremely small. Its diameter ranges between 1.6 fm (for the lightest atom,. How Small Is A Nucleus Compared To An Atom.
From spmscience.blog.onlinetuition.com.my
(A) The composition of the Nucleus SPM Science How Small Is A Nucleus Compared To An Atom Of an atom is less than. The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. The nucleus is tiny compared to the atom as a whole: The nucleus of the atom is extremely small. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen). How Small Is A Nucleus Compared To An Atom.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation How Small Is A Nucleus Compared To An Atom Its radius is only about 1/100,000 of the total radius of the atom. The nucleus of an atom forms its tiny core, with a radius 10,000 to. They are formed of a nucleus, protons, and electrons. Of an atom is less than. The nucleus of the atom is extremely small. It is true the nucleus is a loose cluster of. How Small Is A Nucleus Compared To An Atom.
From www.scienceabc.com
Nucleus Function What Is A Nucleus? What Does The Nucleus Do? How Small Is A Nucleus Compared To An Atom The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by ernest. Atoms make up everything and are incredibly small. To put it another way, an atom the size of a football field has a nucleus the side of a pea. The nucleus is tiny compared to the. How Small Is A Nucleus Compared To An Atom.
From www.slideserve.com
PPT Discovering the Electron PowerPoint Presentation, free download How Small Is A Nucleus Compared To An Atom To put it another way, an atom the size of a football field has a nucleus the side of a pea. They are formed of a nucleus, protons, and electrons. It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the atom. The nucleus is tiny when compared to the size. How Small Is A Nucleus Compared To An Atom.
From slideplayer.com
CHAPTER 2 History of Chemistry. ppt download How Small Is A Nucleus Compared To An Atom The atomic nucleus is extremely tiny, yet very dense. Its radius is only about 1/100,000 of the total radius of the atom. To put it another way, an atom the size of a football field has a nucleus the side of a pea. Atoms make up everything and are incredibly small. The nucleus is tiny when compared to the size. How Small Is A Nucleus Compared To An Atom.
From www.nagwa.com
Question Video Identifying the Expression That Describes the Size of How Small Is A Nucleus Compared To An Atom The atomic nucleus is extremely tiny, yet very dense. Its diameter ranges between 1.6 fm (for the lightest atom, hydrogen) to about 15 fm (for the most massive atoms,. The nucleus is tiny compared to the atom as a whole: It is true the nucleus is a loose cluster of protons and neutrons, but it is tiny compared to the. How Small Is A Nucleus Compared To An Atom.