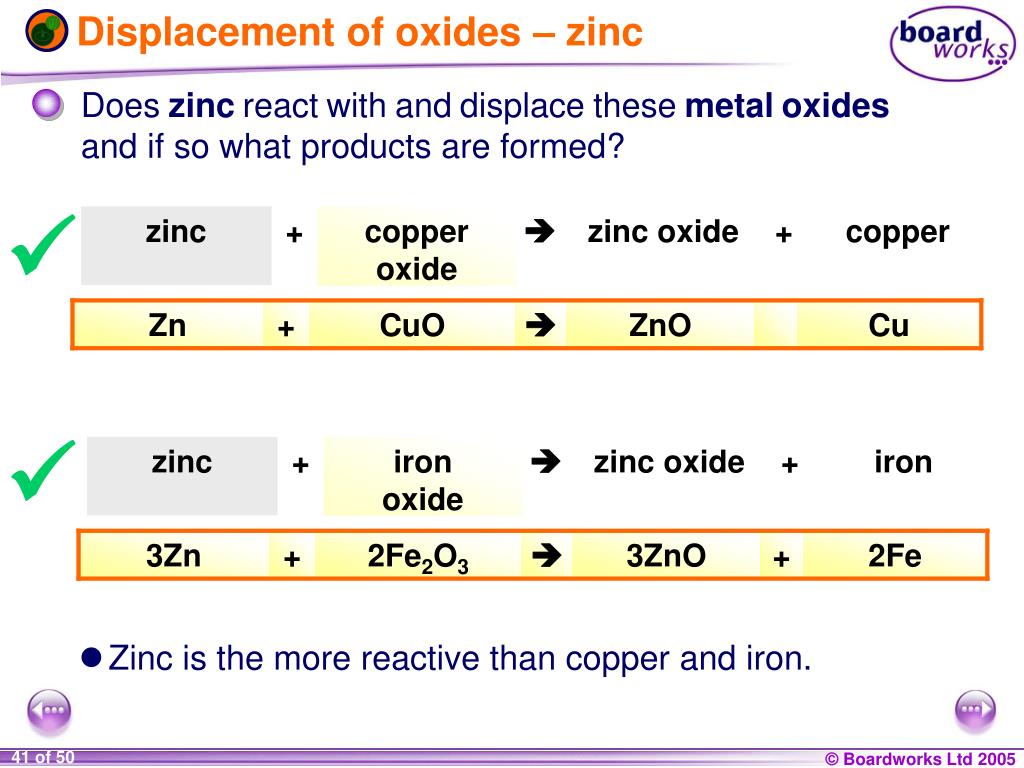
Zinc + Copper Oxide Equation . The reaction between zinc and 42 copper oxide. Aluminium + zinc oxide → aluminium oxide + zinc. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. The general word equation for this reaction is: Metal oxides act as bases. Extracting copper from copper oxide. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of competition reactions. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. I) oxide and zinc metal are reacted together. Metal oxide + carbon → metal + carbon dioxide. Zinc + copper oxide → copper + zinc oxide. Zinc displaces the copper which occurs because zinc is more reactive than copper. Explain which substance is oxidised and which substance acts as an oxidising agent. Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. C and the products can be.
from www.slideserve.com
Metal oxides act as bases. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. The general word equation for this reaction is: Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. I) oxide and zinc metal are reacted together. Metal oxide + carbon → metal + carbon dioxide. Zinc displaces the copper which occurs because zinc is more reactive than copper. The reaction between zinc and 42 copper oxide. C and the products can be. Zinc + copper oxide → copper + zinc oxide.
PPT KS3 Chemistry PowerPoint Presentation, free download ID639260
Zinc + Copper Oxide Equation Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. I) oxide and zinc metal are reacted together. The general word equation for this reaction is: The reaction between zinc and 42 copper oxide. Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Extracting copper from copper oxide. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of competition reactions. Zinc displaces the copper which occurs because zinc is more reactive than copper. Aluminium + zinc oxide → aluminium oxide + zinc. Zinc + copper oxide → copper + zinc oxide. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Metal oxide + carbon → metal + carbon dioxide. Metal oxides act as bases. C and the products can be. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Explain which substance is oxidised and which substance acts as an oxidising agent.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc + Copper Oxide Equation I) oxide and zinc metal are reacted together. Aluminium + zinc oxide → aluminium oxide + zinc. Zinc + copper oxide → copper + zinc oxide. Extracting copper from copper oxide. Zinc displaces the copper which occurs because zinc is more reactive than copper. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole. Zinc + Copper Oxide Equation.
From www.vectorstock.com
Zinc oxide is a molecular chemical formula Vector Image Zinc + Copper Oxide Equation I) oxide and zinc metal are reacted together. Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Extracting copper from copper oxide. Zinc + copper oxide → copper + zinc oxide. The reaction between zinc and 42 copper oxide. Zinc displaces the copper which occurs because zinc is more reactive than copper.. Zinc + Copper Oxide Equation.
From blog.thepipingmart.com
Metallic Oxides of Zinc, Magnesium, and Copper Zinc + Copper Oxide Equation Explain which substance is oxidised and which substance acts as an oxidising agent. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. C and the products can be. To balance a chemical equation, enter an equation of a chemical reaction and. Zinc + Copper Oxide Equation.
From socratic.org
Can you describe the process that releases electrons in a zinc copper Zinc + Copper Oxide Equation Metal oxide + carbon → metal + carbon dioxide. The general word equation for this reaction is: I) oxide and zinc metal are reacted together. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Zinc + copper oxide → copper +. Zinc + Copper Oxide Equation.
From www.vedantu.com
Construct the formula of the following compounds using criss crossing Zinc + Copper Oxide Equation Explain which substance is oxidised and which substance acts as an oxidising agent. Aluminium + zinc oxide → aluminium oxide + zinc. Metal oxide + carbon → metal + carbon dioxide. The reaction between zinc and 42 copper oxide. Extracting copper from copper oxide. Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and. Zinc + Copper Oxide Equation.
From www.chegg.com
Solved look at this word equation. zinc + copper oxide → Zinc + Copper Oxide Equation Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. The reaction between zinc and 42 copper oxide. The general word equation for this reaction is: Zinc displaces the copper which occurs because zinc is more reactive than copper. Explain which substance. Zinc + Copper Oxide Equation.
From studylib.net
Empirical formula of copper oxidestudent Zinc + Copper Oxide Equation Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Metal oxides act as bases. The general word equation for this reaction is: Metal oxide + carbon → metal + carbon dioxide. Aluminium + zinc oxide → aluminium oxide + zinc. Zinc displaces the copper which occurs because zinc is more reactive than. Zinc + Copper Oxide Equation.
From www.alamy.com
Zinc oxide is a molecular chemical formula. Zinc infographics. Vector Zinc + Copper Oxide Equation By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of competition reactions. Metal oxides act as bases. Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Cu + zno = cuo + zn is a single displacement. Zinc + Copper Oxide Equation.
From www.youtube.com
How to write Molecular formula of Zinc OxideChemical formula of Zinc Zinc + Copper Oxide Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zinc displaces the copper which occurs because zinc is more reactive than copper. The general word equation for this reaction is: Aluminium + zinc oxide → aluminium oxide + zinc. C and the products can be. The reaction between zinc and 42 copper. Zinc + Copper Oxide Equation.
From www.gbu-presnenskij.ru
Zinc Oxide Formula Wholesale Cheap www.gbupresnenskij.ru Zinc + Copper Oxide Equation The general word equation for this reaction is: Zinc + copper oxide → copper + zinc oxide. I) oxide and zinc metal are reacted together. Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Aluminium + zinc oxide → aluminium oxide + zinc. C and the products can be. Zinc displaces the. Zinc + Copper Oxide Equation.
From byjus.com
If 1gram of copper on oxidation gives 1.252grams of copper oxide. Find Zinc + Copper Oxide Equation The general word equation for this reaction is: Extracting copper from copper oxide. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of competition reactions. The reaction between zinc and 42 copper oxide. C and the products can be. Copper(ii) oxide and zinc metal react. Zinc + Copper Oxide Equation.
From www.youtube.com
How to Balance Zn(NO3)2 = ZnO + NO2 + O2 (Zinc Nitrate Zinc + Copper Oxide Equation Zinc + copper oxide → copper + zinc oxide. Aluminium + zinc oxide → aluminium oxide + zinc. Metal oxide + carbon → metal + carbon dioxide. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. C and the products can be. The general word equation for this reaction is: Copper(ii) oxide. Zinc + Copper Oxide Equation.
From www.slideserve.com
PPT DO NOW!! PowerPoint Presentation, free download ID1560113 Zinc + Copper Oxide Equation Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. I) oxide and zinc metal are reacted together. The general word equation for this reaction is: Zinc + copper oxide → copper + zinc oxide. Metal oxides act as bases. Metal oxide + carbon → metal + carbon dioxide. To balance a chemical. Zinc + Copper Oxide Equation.
From oneclass.com
CHEM 1211K Lecture Notes Fall 2012, Zinc Sulfate, Chemical Equation Zinc + Copper Oxide Equation Metal oxide + carbon → metal + carbon dioxide. The reaction between zinc and 42 copper oxide. C and the products can be. Aluminium + zinc oxide → aluminium oxide + zinc. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to.. Zinc + Copper Oxide Equation.
From docslib.org
42 the Reaction Between Zinc and Copper Oxide DocsLib Zinc + Copper Oxide Equation Metal oxide + carbon → metal + carbon dioxide. Aluminium + zinc oxide → aluminium oxide + zinc. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of. Zinc + Copper Oxide Equation.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID639260 Zinc + Copper Oxide Equation Aluminium + zinc oxide → aluminium oxide + zinc. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of competition reactions. Explain which substance is oxidised and which substance acts as an oxidising agent. Metal oxide + carbon → metal + carbon dioxide. Zinc +. Zinc + Copper Oxide Equation.
From www.toppr.com
Write balanced chemical equations for the following word equationZinc Zinc + Copper Oxide Equation Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. C and the products can be. Explain which substance is oxidised and which substance acts as an oxidising agent. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea. Zinc + Copper Oxide Equation.
From www.vectorstock.com
Zinc oxide chemical formula Royalty Free Vector Image Zinc + Copper Oxide Equation I) oxide and zinc metal are reacted together. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zinc displaces the copper which occurs because zinc is more reactive than copper. C and the products can be. Zinc + copper oxide → copper + zinc oxide. Extracting copper from copper oxide. Copper(ii) oxide. Zinc + Copper Oxide Equation.
From www.youtube.com
How to Balance Zn + CuSO4 = Cu + ZnSO4 (Zinc plus Copper (II) Sulfate Zinc + Copper Oxide Equation Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Metal oxide + carbon → metal + carbon dioxide. Aluminium + zinc oxide → aluminium oxide + zinc. C and the products can be. The general word equation for this reaction is:. Zinc + Copper Oxide Equation.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Zinc + Copper Oxide Equation Explain which substance is oxidised and which substance acts as an oxidising agent. Metal oxides act as bases. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of competition reactions. I) oxide and zinc metal are reacted together. Aluminium + zinc oxide → aluminium oxide. Zinc + Copper Oxide Equation.
From www.toppr.com
Write balanced chemical equations the following word equationZinc Zinc + Copper Oxide Equation Zinc + copper oxide → copper + zinc oxide. Explain which substance is oxidised and which substance acts as an oxidising agent. Metal oxide + carbon → metal + carbon dioxide. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of competition reactions. Metal oxides. Zinc + Copper Oxide Equation.
From www.sciencecodex.com
Zinc oxide key component for the methanol synthesis reaction over Zinc + Copper Oxide Equation Zinc + copper oxide → copper + zinc oxide. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Metal oxide + carbon → metal. Zinc + Copper Oxide Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc + Copper Oxide Equation By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of competition reactions. Metal oxides act as bases. Metal oxide + carbon → metal + carbon dioxide. The general word equation for this reaction is: Cu + zno = cuo + zn is a single displacement. Zinc + Copper Oxide Equation.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Zinc + Copper Oxide Equation Explain which substance is oxidised and which substance acts as an oxidising agent. Aluminium + zinc oxide → aluminium oxide + zinc. Zinc + copper oxide → copper + zinc oxide. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of competition reactions. Metal oxide. Zinc + Copper Oxide Equation.
From www.meritnation.com
write a word equation for the reaction of Zinc with copper sulphate Zinc + Copper Oxide Equation Extracting copper from copper oxide. The reaction between zinc and 42 copper oxide. Metal oxides act as bases. I) oxide and zinc metal are reacted together. Zinc displaces the copper which occurs because zinc is more reactive than copper. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zinc + copper oxide. Zinc + Copper Oxide Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + CH3COOH = (CH3COO)2Zn + H2 Zinc + Copper Oxide Equation The reaction between zinc and 42 copper oxide. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Extracting copper from copper oxide. Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Metal oxide. Zinc + Copper Oxide Equation.
From www.vrogue.co
Group 2 Metal Reactions A Level Chemistrystudent vrogue.co Zinc + Copper Oxide Equation Extracting copper from copper oxide. Metal oxide + carbon → metal + carbon dioxide. Metal oxides act as bases. Explain which substance is oxidised and which substance acts as an oxidising agent. Zinc displaces the copper which occurs because zinc is more reactive than copper. C and the products can be. Zinc + copper oxide → copper + zinc oxide.. Zinc + Copper Oxide Equation.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc + Copper Oxide Equation The general word equation for this reaction is: C and the products can be. Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Zinc + copper oxide → copper + zinc oxide. Metal oxide + carbon → metal + carbon dioxide. The reaction between zinc and 42 copper oxide. I) oxide and. Zinc + Copper Oxide Equation.
From www.youtube.com
How to Write the Formula for Zinc oxide (ZnO) YouTube Zinc + Copper Oxide Equation Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Metal oxides act as bases. Metal oxide + carbon → metal + carbon dioxide. Copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Explain. Zinc + Copper Oxide Equation.
From resource.studiaacademy.com
Chemical Formulae, Equations and Calculations Studia Academy Resources Zinc + Copper Oxide Equation Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Zinc displaces the copper which occurs because zinc is more reactive than copper. The general word equation for this reaction is: I) oxide and zinc metal are reacted together. To balance a. Zinc + Copper Oxide Equation.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation ID5231819 Zinc + Copper Oxide Equation C and the products can be. The general word equation for this reaction is: The reaction between zinc and 42 copper oxide. Zinc + copper oxide → copper + zinc oxide. Metal oxides act as bases. Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc. Zinc + Copper Oxide Equation.
From byjus.com
how to find the formula of copper oxide Zinc + Copper Oxide Equation The general word equation for this reaction is: Metal oxides act as bases. Zinc displaces the copper which occurs because zinc is more reactive than copper. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Extracting copper from copper oxide. Zinc + copper oxide → copper + zinc oxide. Cu + zno. Zinc + Copper Oxide Equation.
From byjus.com
how to find the formula of copper oxide Zinc + Copper Oxide Equation C and the products can be. Aluminium + zinc oxide → aluminium oxide + zinc. The general word equation for this reaction is: Cu + zno = cuo + zn is a single displacement (substitution) reaction where one mole of copper [cu] and one mole of zinc oxide [zno] react to. Copper(ii) oxide and zinc metal react together in an. Zinc + Copper Oxide Equation.
From www.vectorstock.com
Zinc oxide is a molecular chemical formula Vector Image Zinc + Copper Oxide Equation The reaction between zinc and 42 copper oxide. Metal oxides act as bases. Explain which substance is oxidised and which substance acts as an oxidising agent. The general word equation for this reaction is: Zinc displaces the copper which occurs because zinc is more reactive than copper. To balance a chemical equation, enter an equation of a chemical reaction and. Zinc + Copper Oxide Equation.
From ppt-online.org
Metals презентация онлайн Zinc + Copper Oxide Equation The general word equation for this reaction is: Metal oxides act as bases. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can familiarise themselves with the idea of competition reactions. C and the products can be. Metal oxide + carbon → metal + carbon dioxide. Zinc + copper oxide →. Zinc + Copper Oxide Equation.