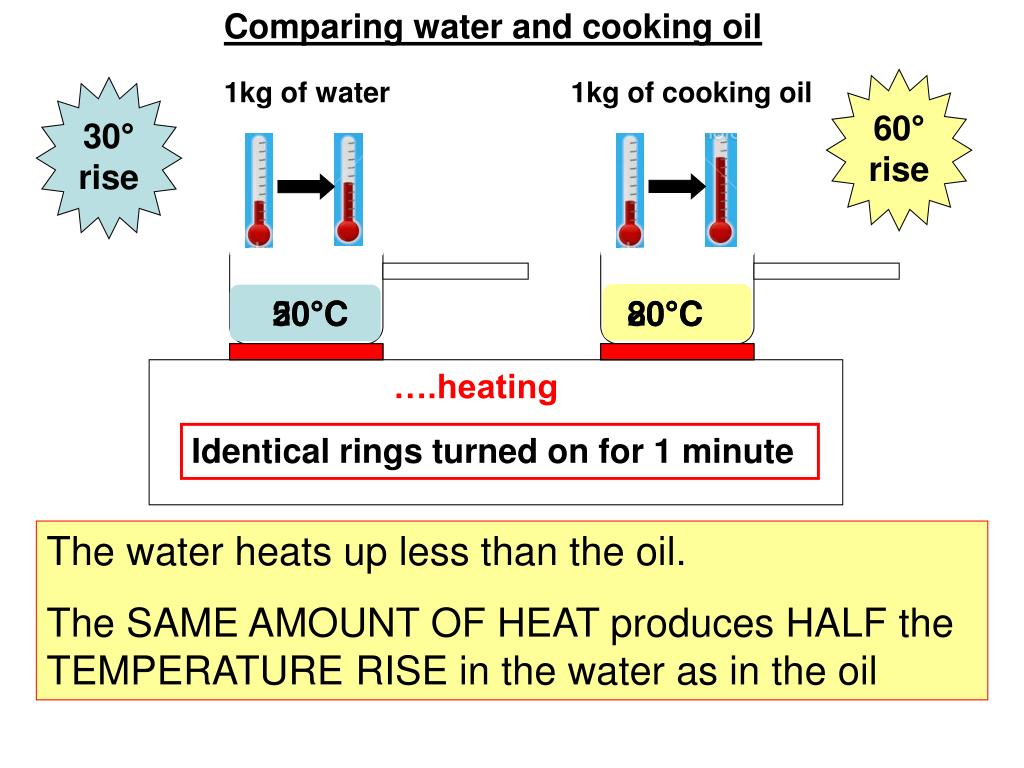
Does Oil Heat Faster Than Water . Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). As it reaches past boiling point of water as the. The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. The heat capacity of oil is about half that of water. That is due to the difference in specific heat capacities. The oil will get hotter than the water for a given amount of added heat. Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual hydrogen bonding per. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. Oil heats up faster than water because it has a lower specific heat capacity.
from learningschoolmaharaja.z14.web.core.windows.net
The oil will get hotter than the water for a given amount of added heat. Oil heats up faster than water because it has a lower specific heat capacity. The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. That is due to the difference in specific heat capacities. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual hydrogen bonding per. As it reaches past boiling point of water as the. The heat capacity of oil is about half that of water.
Cooking Oil Heat Capacity
Does Oil Heat Faster Than Water Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual hydrogen bonding per. The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. The oil will get hotter than the water for a given amount of added heat. The heat capacity of oil is about half that of water. Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual hydrogen bonding per. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). As it reaches past boiling point of water as the. That is due to the difference in specific heat capacities. Oil heats up faster than water because it has a lower specific heat capacity.
From www.youtube.com
Effects of Heat on Water YouTube Does Oil Heat Faster Than Water The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. That is due to the difference in specific heat capacities. The heat capacity of oil is about half that of water. Oil is thought of as hotter because it can be heated to higher. Does Oil Heat Faster Than Water.
From www.slideshare.net
More heat Does Oil Heat Faster Than Water As it reaches past boiling point of water as the. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Oil is thought of as hotter because it can be heated to higher temperatures than boiling. The oil will get hotter than the water for a. Does Oil Heat Faster Than Water.
From www.youtube.com
To show that land gets heated up faster than water and loses heat Does Oil Heat Faster Than Water That is due to the difference in specific heat capacities. The oil will get hotter than the water for a given amount of added heat. Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual hydrogen bonding per. Oil heats up faster than water because it has a lower specific heat capacity.. Does Oil Heat Faster Than Water.
From www.iqsdirectory.com
Shell and Tube Heat Exchanger What Is It? Types, Process Does Oil Heat Faster Than Water Oil heats up faster than water because it has a lower specific heat capacity. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. That is due to the difference in specific heat capacities. The lower specific heat of oil means that it is easier to heat or cool than water, making if more. Does Oil Heat Faster Than Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Does Oil Heat Faster Than Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Oil heats up faster than water because it has a lower specific heat capacity. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. The lower specific heat of oil. Does Oil Heat Faster Than Water.
From masterconceptsinchemistry.com
How does the vapor pressure of solution depend on the concentration of Does Oil Heat Faster Than Water That is due to the difference in specific heat capacities. Oil heats up faster than water because it has a lower specific heat capacity. As it reaches past boiling point of water as the. The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a.. Does Oil Heat Faster Than Water.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board Does Oil Heat Faster Than Water Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual hydrogen bonding per. The oil will get hotter than the water for a given amount of added heat. The heat capacity of oil is about half that of water. As it reaches past boiling point of water as the. Specific heat is. Does Oil Heat Faster Than Water.
From www.slideserve.com
PPT Earth’s Temperature, Concepts, and Patterns PowerPoint Does Oil Heat Faster Than Water The oil will get hotter than the water for a given amount of added heat. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Oil is thought of as hotter because it can be heated to higher temperatures than boiling. As it reaches past boiling. Does Oil Heat Faster Than Water.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition Does Oil Heat Faster Than Water As it reaches past boiling point of water as the. The heat capacity of oil is about half that of water. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Water has a high heat capacity and can absorb/trap alot of heat due to its. Does Oil Heat Faster Than Water.
From letstalkscience.ca
Introduction to Heat Transfer Let's Talk Science Does Oil Heat Faster Than Water That is due to the difference in specific heat capacities. Oil heats up faster than water because it has a lower specific heat capacity. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). The oil will get hotter than the water for a given amount. Does Oil Heat Faster Than Water.
From colosoimages.com
convection currents labeled diagram Coloso Does Oil Heat Faster Than Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Oil is thought of as hotter because it can be heated to higher temperatures than boiling. That is due to the difference in specific heat capacities. Oil heats up faster than water because it has a. Does Oil Heat Faster Than Water.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Does Oil Heat Faster Than Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). The oil will get hotter than the water for a given amount of added heat. Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual hydrogen bonding per.. Does Oil Heat Faster Than Water.
From physicsexperiments.eu
Comparing Specific Heat of Water and Vegetable Oil — Collection of Does Oil Heat Faster Than Water Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual hydrogen bonding per. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. The heat capacity of oil is about half that of water. That is due to the difference in specific heat capacities. Oil heats. Does Oil Heat Faster Than Water.
From thewaterfiltermarket.com
How Long Does It Take for Water To Evaporate? Water Filter Market Does Oil Heat Faster Than Water Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual hydrogen bonding per. The heat capacity of oil is about half that of water. Oil heats up faster than water because it has a lower specific heat capacity. As it reaches past boiling point of water as the. That is due to. Does Oil Heat Faster Than Water.
From www.slideserve.com
PPT How do particles behave in the four states of matter? PowerPoint Does Oil Heat Faster Than Water Oil is thought of as hotter because it can be heated to higher temperatures than boiling. The oil will get hotter than the water for a given amount of added heat. Oil heats up faster than water because it has a lower specific heat capacity. Specific heat is defined by the amount of heat needed to raise the temperature of. Does Oil Heat Faster Than Water.
From www.slideshare.net
Meteorology Does Oil Heat Faster Than Water That is due to the difference in specific heat capacities. The heat capacity of oil is about half that of water. As it reaches past boiling point of water as the. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. Specific heat is defined by the amount of heat needed to raise the. Does Oil Heat Faster Than Water.
From learningschoolmaharaja.z14.web.core.windows.net
Cooking Oil Heat Capacity Does Oil Heat Faster Than Water As it reaches past boiling point of water as the. That is due to the difference in specific heat capacities. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Water has a high heat capacity and can absorb/trap alot of heat due to its ability. Does Oil Heat Faster Than Water.
From inspectapedia.com
Hot Water Heating Boiler Operation Details 39 steps in hydronic Does Oil Heat Faster Than Water The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. Oil heats up faster than water because it has a lower specific heat capacity. That is due to the difference in specific heat capacities. Water has a high heat capacity and can absorb/trap alot. Does Oil Heat Faster Than Water.
From oilheatgurokugi.blogspot.com
Oil Heat Why Does Oil Heat Faster Than Water Does Oil Heat Faster Than Water The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. As it reaches past boiling point of water as the. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c).. Does Oil Heat Faster Than Water.
From www.researchgate.net
Heat capacity of crude oil vs. Temperature Download Scientific Diagram Does Oil Heat Faster Than Water Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual hydrogen bonding per. The heat capacity of oil is about half that of water. That is due to the difference in specific heat capacities. Oil heats up faster than water because it has a lower specific heat capacity. Oil is thought of. Does Oil Heat Faster Than Water.
From www.pinterest.com
How thermals form. Science diagram showing how molecules react during Does Oil Heat Faster Than Water The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. The oil will get hotter than the water for a given amount of added heat. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. Specific heat is. Does Oil Heat Faster Than Water.
From www.geeksforgeeks.org
Heat Capacity Does Oil Heat Faster Than Water The oil will get hotter than the water for a given amount of added heat. As it reaches past boiling point of water as the. Oil heats up faster than water because it has a lower specific heat capacity. That is due to the difference in specific heat capacities. Specific heat is defined by the amount of heat needed to. Does Oil Heat Faster Than Water.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Does Oil Heat Faster Than Water As it reaches past boiling point of water as the. The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. The heat capacity of oil is about half that of water. Oil is thought of as hotter because it can be heated to higher. Does Oil Heat Faster Than Water.
From www.winkelhage.com
Water Systems Mr. Winkelhage's site Does Oil Heat Faster Than Water That is due to the difference in specific heat capacities. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). The oil will get hotter than the water for a given amount of added heat. Oil heats up faster than water because it has a lower. Does Oil Heat Faster Than Water.
From www.youtube.com
Boiling point of oil is greater than water ? YouTube Does Oil Heat Faster Than Water Oil is thought of as hotter because it can be heated to higher temperatures than boiling. The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. Water has a high heat capacity and can absorb/trap alot of heat due to its ability of dual. Does Oil Heat Faster Than Water.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download Does Oil Heat Faster Than Water That is due to the difference in specific heat capacities. The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. Oil heats up faster than water because it. Does Oil Heat Faster Than Water.
From www.youtube.com
Does Cold or Hot Water Boil Faster? YouTube Does Oil Heat Faster Than Water The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). As it reaches past boiling point of water as the.. Does Oil Heat Faster Than Water.
From blog.smarttouchenergy.com
How Your Oil Heating System Works Does Oil Heat Faster Than Water Oil heats up faster than water because it has a lower specific heat capacity. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. That is due to. Does Oil Heat Faster Than Water.
From www.numerade.com
SOLVED Hot oil is to be cooled in a multipass shellandtube heat Does Oil Heat Faster Than Water Oil is thought of as hotter because it can be heated to higher temperatures than boiling. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). As it reaches past boiling point of water as the. The lower specific heat of oil means that it is. Does Oil Heat Faster Than Water.
From smoenergy.com
How Does Oil Heat My Home? SMO Energy Does Oil Heat Faster Than Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). The heat capacity of oil is about half that of water. That is due to the difference in specific heat capacities. The oil will get hotter than the water for a given amount of added heat.. Does Oil Heat Faster Than Water.
From www.pinterest.com
How temperature affects Diffusion. Full Demonstration and Explanation Does Oil Heat Faster Than Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. Oil heats up faster than water because it has a. Does Oil Heat Faster Than Water.
From www.youtube.com
Oil in Water vs Water in Oil Emulsions Fast differences and Comparison Does Oil Heat Faster Than Water The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. Oil heats up faster than water because it has a lower specific heat capacity. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. The oil will get. Does Oil Heat Faster Than Water.
From www.slideshare.net
Ch 16 Wind And Water Does Oil Heat Faster Than Water The oil will get hotter than the water for a given amount of added heat. The lower specific heat of oil means that it is easier to heat or cool than water, making if more efficient at transferring energy from a. That is due to the difference in specific heat capacities. Oil heats up faster than water because it has. Does Oil Heat Faster Than Water.
From wt.kimiq.com
Heating Curve Of Water Water Ionizer Does Oil Heat Faster Than Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Oil heats up faster than water because it has a lower specific heat capacity. Oil is thought of as hotter because it can be heated to higher temperatures than boiling. As it reaches past boiling point. Does Oil Heat Faster Than Water.
From www.youtube.com
Oil Heat 🔥 Boilers How it works Understand the Basics YouTube Does Oil Heat Faster Than Water Oil is thought of as hotter because it can be heated to higher temperatures than boiling. Oil heats up faster than water because it has a lower specific heat capacity. That is due to the difference in specific heat capacities. The lower specific heat of oil means that it is easier to heat or cool than water, making if more. Does Oil Heat Faster Than Water.